Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018
JAMA ( IF 63.1 ) Pub Date : 2020-03-03 , DOI: 10.1001/jama.2020.1166 Olivier J Wouters 1 , Martin McKee 2 , Jeroen Luyten 3
JAMA ( IF 63.1 ) Pub Date : 2020-03-03 , DOI: 10.1001/jama.2020.1166 Olivier J Wouters 1 , Martin McKee 2 , Jeroen Luyten 3
Affiliation
|
Importance
The mean cost of developing a new drug has been the subject of debate, with recent estimates ranging from $314 million to $2.8 billion. Objective
To estimate the research and development investment required to bring a new therapeutic agent to market, using publicly available data. Design and Setting
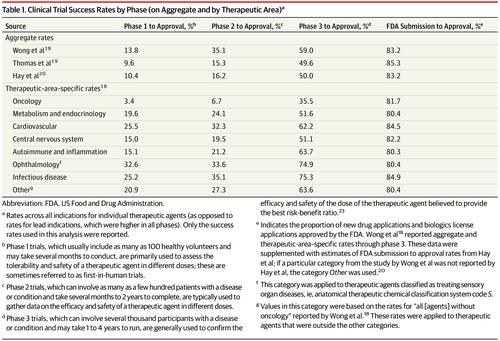
Data were analyzed on new therapeutic agents approved by the US Food and Drug Administration (FDA) between 2009 and 2018 to estimate the research and development expenditure required to bring a new medicine to market. Data were accessed from the US Securities and Exchange Commission, Drugs@FDA database, and ClinicalTrials.gov, alongside published data on clinical trial success rates. Exposures
Conduct of preclinical and clinical studies of new therapeutic agents. Main Outcomes and Measures
Median and mean research and development spending on new therapeutic agents approved by the FDA, capitalized at a real cost of capital rate (the required rate of return for an investor) of 10.5% per year, with bootstrapped CIs. All amounts were reported in 2018 US dollars. Results
The FDA approved 355 new drugs and biologics over the study period. Research and development expenditures were available for 63 (18%) products, developed by 47 different companies. After accounting for the costs of failed trials, the median capitalized research and development investment to bring a new drug to market was estimated at $985.3 million (95% CI, $683.6 million-$1228.9 million), and the mean investment was estimated at $1335.9 million (95% CI, $1042.5 million-$1637.5 million) in the base case analysis. Median estimates by therapeutic area (for areas with ≥5 drugs) ranged from $765.9 million (95% CI, $323.0 million-$1473.5 million) for nervous system agents to $2771.6 million (95% CI, $2051.8 million-$5366.2 million) for antineoplastic and immunomodulating agents. Data were mainly accessible for smaller firms, orphan drugs, products in certain therapeutic areas, first-in-class drugs, therapeutic agents that received accelerated approval, and products approved between 2014 and 2018. Results varied in sensitivity analyses using different estimates of clinical trial success rates, preclinical expenditures, and cost of capital. Conclusions and Relevance
This study provides an estimate of research and development costs for new therapeutic agents based on publicly available data. Differences from previous studies may reflect the spectrum of products analyzed, the restricted availability of data in the public domain, and differences in underlying assumptions in the cost calculations.
中文翻译:

2009-2018 年将新药推向市场所需的预计研发投资
重要性 开发新药的平均成本一直是争论的话题,最近的估计在 3.14 亿美元到 28 亿美元之间。目的 使用公开数据估算将新治疗药物推向市场所需的研发投资。对 2009 年至 2018 年间美国食品和药物管理局 (FDA) 批准的新治疗药物的设计和设置数据进行了分析,以估算将新药推向市场所需的研发支出。数据来自美国证券交易委员会、Drugs@FDA 数据库和 ClinicalTrials.gov,以及已发布的临床试验成功率数据。暴露 进行新治疗药物的临床前和临床研究。主要成果和措施 FDA 批准的新治疗药物的中位和平均研发支出,以每年 10.5% 的实际资本成本率(投资者所需的回报率)资本化,并采用自举 CI。所有金额均以 2018 年美元报告。结果 FDA 在研究期间批准了 355 种新药和生物制剂。研发支出可用于 47 家不同公司开发的 63 种产品(18%)。考虑到试验失败的成本后,将新药推向市场的资本化研发投资中位数估计为 9.853 亿美元(95% CI,6.836 亿美元至 12.289 亿美元),平均投资估计为 13.359 亿美元( 95% CI,10.425 亿美元至 16.375 亿美元)在基本案例分析中。按治疗领域(≥5 种药物的领域)估计的中位值从神经系统药物的 7.659 亿美元(95% CI,3.23 亿至 14.735 亿美元)到 27.716 亿美元(95% CI,2051 年)不等。800万-53.662亿美元)用于抗肿瘤药物和免疫调节药物。数据主要针对较小的公司、孤儿药、某些治疗领域的产品、一流药物、获得加速批准的治疗药物以及 2014 年至 2018 年期间批准的产品。使用不同的临床试验估计进行敏感性分析,结果各不相同成功率、临床前支出和资本成本。结论和相关性 本研究根据公开数据对新治疗药物的研发成本进行了估算。与之前研究的差异可能反映了分析的产品范围、公共领域数据的有限可用性以及成本计算中基本假设的差异。
更新日期:2020-03-03
中文翻译:

2009-2018 年将新药推向市场所需的预计研发投资
重要性 开发新药的平均成本一直是争论的话题,最近的估计在 3.14 亿美元到 28 亿美元之间。目的 使用公开数据估算将新治疗药物推向市场所需的研发投资。对 2009 年至 2018 年间美国食品和药物管理局 (FDA) 批准的新治疗药物的设计和设置数据进行了分析,以估算将新药推向市场所需的研发支出。数据来自美国证券交易委员会、Drugs@FDA 数据库和 ClinicalTrials.gov,以及已发布的临床试验成功率数据。暴露 进行新治疗药物的临床前和临床研究。主要成果和措施 FDA 批准的新治疗药物的中位和平均研发支出,以每年 10.5% 的实际资本成本率(投资者所需的回报率)资本化,并采用自举 CI。所有金额均以 2018 年美元报告。结果 FDA 在研究期间批准了 355 种新药和生物制剂。研发支出可用于 47 家不同公司开发的 63 种产品(18%)。考虑到试验失败的成本后,将新药推向市场的资本化研发投资中位数估计为 9.853 亿美元(95% CI,6.836 亿美元至 12.289 亿美元),平均投资估计为 13.359 亿美元( 95% CI,10.425 亿美元至 16.375 亿美元)在基本案例分析中。按治疗领域(≥5 种药物的领域)估计的中位值从神经系统药物的 7.659 亿美元(95% CI,3.23 亿至 14.735 亿美元)到 27.716 亿美元(95% CI,2051 年)不等。800万-53.662亿美元)用于抗肿瘤药物和免疫调节药物。数据主要针对较小的公司、孤儿药、某些治疗领域的产品、一流药物、获得加速批准的治疗药物以及 2014 年至 2018 年期间批准的产品。使用不同的临床试验估计进行敏感性分析,结果各不相同成功率、临床前支出和资本成本。结论和相关性 本研究根据公开数据对新治疗药物的研发成本进行了估算。与之前研究的差异可能反映了分析的产品范围、公共领域数据的有限可用性以及成本计算中基本假设的差异。











































 京公网安备 11010802027423号
京公网安备 11010802027423号