当前位置:
X-MOL 学术
›
J. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phase Separation and Disorder-to-Order Transition of Human Brain Expressed X-Linked 3 (hBEX3) in the Presence of Small Fragments of tRNA.
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.jmb.2020.02.030 Mariana J do Amaral 1 , Talita S Araujo 1 , Nuria C Díaz 2 , Federica Accornero 3 , Carla R Polycarpo 1 , Yraima Cordeiro 2 , Katia M S Cabral 4 , Marcius S Almeida 5
Journal of Molecular Biology ( IF 4.7 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.jmb.2020.02.030 Mariana J do Amaral 1 , Talita S Araujo 1 , Nuria C Díaz 2 , Federica Accornero 3 , Carla R Polycarpo 1 , Yraima Cordeiro 2 , Katia M S Cabral 4 , Marcius S Almeida 5
Affiliation
|
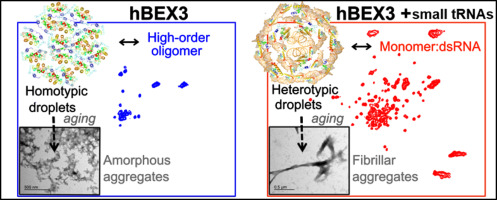
Brain Expressed X-linked (BEX) protein family consists of five members in humans and is highly expressed during neuronal development. They are known to participate in cell cycle and in signaling pathways involved in neurodegeneration and cancer. BEX3 possess a conserved leucine-rich nuclear export signal and experimental data confirmed BEX3 nucleocytoplasmic shuttling. Previous data revealed that mouse BEX3 auto-associates in an oligomer rich in intrinsic disorder. In this work, we show that human BEX3 (hBEX3) has well-defined three-dimensional structure in the presence of small fragments of tRNA (tRFs). Conversely, the nucleic acids-free purified hBEX3 presented disordered structure. Small-angle X-ray scattering data revealed that in the presence of tRFs, hBEX3 adopts compact globular fold, which is very distinct from the elongated high-order oligomer formed by the pure protein. Furthermore, microscopy showed that hBEX3 undergoes condensation in micron-sized protein-rich droplets in vitro. In the presence of tRFs, biomolecular condensates were smaller and in higher number, showing acridine orange green fluorescence emission, which corroborated with the presence of base-paired nucleic acids. Additionally, we found that over time hBEX3 transits from liquid condensates to aggregates that are reversible upon temperature increment and dissolved by 1,6-hexanediol. hBEX3 assemblies display different morphology in the presence of the tRFs that seems to protect from amyloid formation. Collectively, our findings support a role for tRFs in hBEX3 disorder-to-order transition and modulation of phase transitions. Moreover, hBEX3 aggregation-prone features and the specificity in interaction with tRNA fragments advocate paramount importance toward understanding BEX family involvement in neurodevelopment and cell death.
中文翻译:

在存在tRNA小片段的情况下,人脑表达的X连锁3(hBEX3)的相分离和无序转换。
脑表达X连锁(BEX)蛋白家族由人类的五个成员组成,并在神经元发育过程中高度表达。已知它们参与细胞周期以及参与神经变性和癌症的信号通路。BEX3具有保守的富含亮氨酸的核输出信号,实验数据证实了BEX3的核质穿梭。先前的数据显示,小鼠BEX3在富含内源性疾病的寡聚体中自动缔合。在这项工作中,我们表明人BEX3(hBEX3)在存在tRNA小片段(tRF)的情况下具有定义明确的三维结构。相反,无核酸的纯化hBEX3呈现无序结构。小角度X射线散射数据表明,在存在tRF的情况下,hBEX3具有紧密的球状褶皱,这与纯蛋白质形成的细长的高阶低聚物截然不同。此外,显微镜显示hBEX3在体外会在微米大小的富含蛋白质的液滴中凝结。在存在tRF的情况下,生物分子缩合物较小且数目较高,显示出idine啶橙绿色荧光发射,这与碱基配对核酸的存在相符。此外,我们发现,随着时间的流逝,hBEX3从液态冷凝物转变为聚集体,聚集体在温度升高时可逆,并被1,6-己二醇溶解。hBEX3组件在tRF存在下显示出不同的形态,似乎可以防止淀粉样蛋白形成。总体而言,我们的发现支持tRF在hBEX3无序转移和相变调制中的作用。此外,
更新日期:2020-03-03
中文翻译:

在存在tRNA小片段的情况下,人脑表达的X连锁3(hBEX3)的相分离和无序转换。
脑表达X连锁(BEX)蛋白家族由人类的五个成员组成,并在神经元发育过程中高度表达。已知它们参与细胞周期以及参与神经变性和癌症的信号通路。BEX3具有保守的富含亮氨酸的核输出信号,实验数据证实了BEX3的核质穿梭。先前的数据显示,小鼠BEX3在富含内源性疾病的寡聚体中自动缔合。在这项工作中,我们表明人BEX3(hBEX3)在存在tRNA小片段(tRF)的情况下具有定义明确的三维结构。相反,无核酸的纯化hBEX3呈现无序结构。小角度X射线散射数据表明,在存在tRF的情况下,hBEX3具有紧密的球状褶皱,这与纯蛋白质形成的细长的高阶低聚物截然不同。此外,显微镜显示hBEX3在体外会在微米大小的富含蛋白质的液滴中凝结。在存在tRF的情况下,生物分子缩合物较小且数目较高,显示出idine啶橙绿色荧光发射,这与碱基配对核酸的存在相符。此外,我们发现,随着时间的流逝,hBEX3从液态冷凝物转变为聚集体,聚集体在温度升高时可逆,并被1,6-己二醇溶解。hBEX3组件在tRF存在下显示出不同的形态,似乎可以防止淀粉样蛋白形成。总体而言,我们的发现支持tRF在hBEX3无序转移和相变调制中的作用。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号