当前位置:
X-MOL 学术
›
Mol. Ther.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
CD8+ T Cells Impact Rising PSA in Biochemically Relapsed Cancer Patients Using Immunotherapy Targeting Tumor-Associated Antigens.
Molecular Therapy ( IF 12.4 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.ymthe.2020.02.018 Neal D Shore 1 , Matthew P Morrow 2 , Trevor McMullan 2 , Kimberly A Kraynyak 2 , Albert Sylvester 2 , Khamal Bhatt 2 , Jocelyn Cheung 2 , Jean D Boyer 2 , Li Liu 2 , Brian Sacchetta 2 , Samantha Rosencranz 2 , Elizabeth I Heath 3 , Luke Nordquist 4 , Heather H Cheng 5 , Scott T Tagawa 6 , Leonard J Appleman 7 , Ronald Tutrone 8 , Jorge A Garcia 9 , Young E Whang 10 , W Kevin Kelly 11 , David B Weiner 12 , Mark L Bagarazzi 2 , Jeffrey M Skolnik 2
Molecular Therapy ( IF 12.4 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.ymthe.2020.02.018 Neal D Shore 1 , Matthew P Morrow 2 , Trevor McMullan 2 , Kimberly A Kraynyak 2 , Albert Sylvester 2 , Khamal Bhatt 2 , Jocelyn Cheung 2 , Jean D Boyer 2 , Li Liu 2 , Brian Sacchetta 2 , Samantha Rosencranz 2 , Elizabeth I Heath 3 , Luke Nordquist 4 , Heather H Cheng 5 , Scott T Tagawa 6 , Leonard J Appleman 7 , Ronald Tutrone 8 , Jorge A Garcia 9 , Young E Whang 10 , W Kevin Kelly 11 , David B Weiner 12 , Mark L Bagarazzi 2 , Jeffrey M Skolnik 2
Affiliation
|
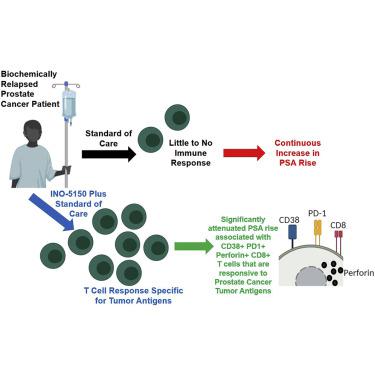
The management of men with prostate cancer (PCa) with biochemical recurrence following local definitive therapy remains controversial. Early use of androgen deprivation therapy (ADT) leads to significant side effects. Developing an alternative, clinically effective, and well-tolerated therapy remains an unmet clinical need. INO-5150 is a synthetic DNA therapy that includes plasmids encoding for prostate-specific antigen (PSA) and prostate-specific membrane antigen (PSMA), and INO-9012 is a synthetic DNA plasmid encoding for interleukin-12 (IL-12). This phase 1/2, open-label, multi-center study enrolled men with PCa with rising PSA after surgery and/or radiation therapy. Patients were enrolled into one of four treatment arms: arm A, 2 mg of INO-5150; arm B, 8.5 mg of INO-5150; arm C, 2 mg of INO-5150 + 1 mg of INO-9012; and arm D, 8.5 mg of INO-5150 + 1 mg of INO-9012. Patients received study drug with electroporation on day 0 and on weeks 3, 12, and 24, and they were followed for up to 72 weeks. Sixty-two patients were enrolled. Treatment was well tolerated. 81% (50/62) of patients completed all visits. 85% (53/62) remained progression-free at 72 weeks. PSA doubling time (PSADT) was increased when assessed in patients with day 0 PSADT ≤12 months. Immunogenicity was observed in 76% (47/62) of patients by multiple assessments. Analysis indicated that CD38 and perforin co-positive CD8 T cell frequency correlated with attenuated PSA rise (p = 0.05, n = 50).
中文翻译:

使用靶向肿瘤相关抗原的免疫疗法,CD8 + T细胞影响生化复发癌症患者中PSA的升高。
局部明确治疗后生化复发的前列腺癌(PCa)男性的治疗仍存在争议。早期使用雄激素剥夺疗法(ADT)会导致严重的副作用。开发替代的,临床有效的和耐受性良好的疗法仍然是未满足的临床需求。INO-5150是一种合成DNA疗法,包括编码前列腺特异性抗原(PSA)和前列腺特异性膜抗原(PSMA)的质粒,而INO-9012是一种编码白介素12(IL-12)的合成DNA质粒。该1/2期,开放标签,多中心研究招募了接受PCa手术和/或放射治疗后PSA升高的男性。将患者纳入四个治疗组之一:A组,2 mg INO-5150;B组,8.5 mg INO-5150;C臂,2 mg INO-5150 + 1 mg INO-9012;和手臂D,8。5毫克INO-5150 + 1毫克INO-9012。患者在第0天以及第3、12和24周接受了带有电穿孔的研究药物,并接受了长达72周的随访。招募了62名患者。治疗耐受性良好。81%(50/62)的患者完成了所有就诊。在72周时,仍有85%(53/62)的患者无进展。在第0天PSADT≤12个月的患者中评估时,PSA加倍时间(PSADT)增加。通过多次评估,在76%(47/62)的患者中观察到了免疫原性。分析表明,CD38和穿孔素共阳性的CD8 T细胞频率与PSA升高的衰减相关(p = 0.05,n = 50)。81%(50/62)的患者完成了所有就诊。在72周时,仍有85%(53/62)的患者无进展。在第0天PSADT≤12个月的患者中评估时,PSA加倍时间(PSADT)增加。通过多次评估,在76%(47/62)的患者中观察到了免疫原性。分析表明,CD38和穿孔素共阳性的CD8 T细胞频率与PSA升高的衰减相关(p = 0.05,n = 50)。81%(50/62)的患者完成了所有就诊。在72周时,仍有85%(53/62)的患者无进展。在第0天PSADT≤12个月的患者中评估时,PSA加倍时间(PSADT)增加。通过多次评估,在76%(47/62)的患者中观察到了免疫原性。分析表明,CD38和穿孔素共阳性的CD8 T细胞频率与PSA升高的衰减相关(p = 0.05,n = 50)。
更新日期:2020-03-03
中文翻译:

使用靶向肿瘤相关抗原的免疫疗法,CD8 + T细胞影响生化复发癌症患者中PSA的升高。
局部明确治疗后生化复发的前列腺癌(PCa)男性的治疗仍存在争议。早期使用雄激素剥夺疗法(ADT)会导致严重的副作用。开发替代的,临床有效的和耐受性良好的疗法仍然是未满足的临床需求。INO-5150是一种合成DNA疗法,包括编码前列腺特异性抗原(PSA)和前列腺特异性膜抗原(PSMA)的质粒,而INO-9012是一种编码白介素12(IL-12)的合成DNA质粒。该1/2期,开放标签,多中心研究招募了接受PCa手术和/或放射治疗后PSA升高的男性。将患者纳入四个治疗组之一:A组,2 mg INO-5150;B组,8.5 mg INO-5150;C臂,2 mg INO-5150 + 1 mg INO-9012;和手臂D,8。5毫克INO-5150 + 1毫克INO-9012。患者在第0天以及第3、12和24周接受了带有电穿孔的研究药物,并接受了长达72周的随访。招募了62名患者。治疗耐受性良好。81%(50/62)的患者完成了所有就诊。在72周时,仍有85%(53/62)的患者无进展。在第0天PSADT≤12个月的患者中评估时,PSA加倍时间(PSADT)增加。通过多次评估,在76%(47/62)的患者中观察到了免疫原性。分析表明,CD38和穿孔素共阳性的CD8 T细胞频率与PSA升高的衰减相关(p = 0.05,n = 50)。81%(50/62)的患者完成了所有就诊。在72周时,仍有85%(53/62)的患者无进展。在第0天PSADT≤12个月的患者中评估时,PSA加倍时间(PSADT)增加。通过多次评估,在76%(47/62)的患者中观察到了免疫原性。分析表明,CD38和穿孔素共阳性的CD8 T细胞频率与PSA升高的衰减相关(p = 0.05,n = 50)。81%(50/62)的患者完成了所有就诊。在72周时,仍有85%(53/62)的患者无进展。在第0天PSADT≤12个月的患者中评估时,PSA加倍时间(PSADT)增加。通过多次评估,在76%(47/62)的患者中观察到了免疫原性。分析表明,CD38和穿孔素共阳性的CD8 T细胞频率与PSA升高的衰减相关(p = 0.05,n = 50)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号