Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 3.4 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.bbamem.2020.183242 Sumana Srinivasan 1 , Faiza Hanif Waghu 2 , Susan Idicula-Thomas 3 , Kareenhalli V Venkatesh 1
|
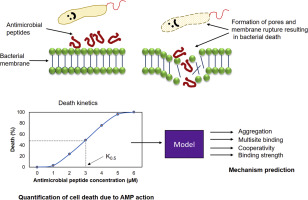
Antimicrobial Peptides (AMPs) are host defense molecules that initiate microbial death by binding to the membrane. On membrane binding, AMPs undergo changes in conformation and aggregation state to enable killing action. Depending on the AMP and cell membrane characteristics, the nature of binding can be aggregating or non-aggregating, with high/low cooperativity, at single or multiple sites with high/low affinity leading to a unique killing action that needs to be studied individually. In the present study, a steady-state model that simulates AMP-membrane interaction was developed and was used to predict the mechanism of AMP binding. The predictions obtained from the model were validated with experimentally deciphered values available in literature. The model was further used to predict the mechanism for a set of designed AMPs with high sequence similarity to Myeloid Antimicrobial Peptide (MAP) family. Depending on the predicted mechanism, a unique half saturation constant and steepness of response (Hill coefficient) was obtained which was further validated with available data from literature. The model could reliably predict the mechanism, the half saturation constant and the Hill coefficient values. Further based on the analysis, it was observed that aggregation and oligomerization result in drastic killing action in a short range of peptide concentration owing to high Hill coefficient values. Mechanisms such as monomers binding at multiple sites with/without cooperativity result in antimicrobial activity at low half saturation constant though the killing action may not be steep. Thus, the methodology developed here can be used to develop hypothesis for studying AMP-membrane interaction mechanisms.
中文翻译:

用于模拟抗菌肽-细胞膜相互作用的稳态建模方法。
抗菌肽(AMPs)是宿主防御分子,通过与膜结合而引发微生物死亡。在膜结合时,AMP经历构象和聚集状态的变化以实现杀伤作用。根据AMP和细胞膜的特性,结合的性质可以在单个或多个具有高/低亲和力的位点以高/低协同性聚集或不聚集,从而导致独特的杀伤作用,需要单独研究。在本研究中,建立了一个模拟AMP-膜相互作用的稳态模型,并用于预测AMP结合的机制。从模型中获得的预测已通过文献中提供的实验破译值进行了验证。该模型还用于预测一组设计的与骨髓抗微生物肽(MAP)家族具有高度序列相似性的AMP的机制。根据预测的机理,获得了独特的半饱和常数和响应陡度(希尔系数),并用文献中的可用数据进行了进一步验证。该模型可以可靠地预测机理,半饱和常数和希尔系数值。进一步基于该分析,观察到由于高希尔系数值,聚集和低聚导致在短肽浓度范围内的剧烈杀伤作用。尽管杀灭作用可能并不陡峭,但是诸如单体在多个位点结合有/没有协同作用的机制导致了低半饱和常数的抗菌活性。从而,



























 京公网安备 11010802027423号
京公网安备 11010802027423号