European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.ejmech.2020.112201 Ling-Ling Yang , Hua-Li Wang , Yu-Hang Yan , Sha Liu , Zhu-Jun Yu , Meng-Yi Huang , Yubin Luo , Xi Zheng , Yamei Yu , Guo-Bo Li
|
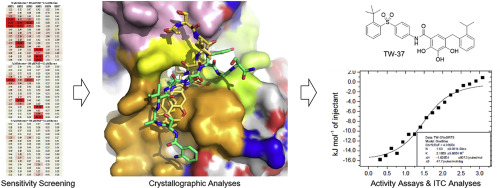
Sirtuins (SIRTs) are NAD+-dependent lysine deacylases, regulating many important biological processes such as metabolism and stress responses. SIRT inhibitors may provide potential benefits against SIRT-driven human diseases. Development of efficient assay platforms based on fluorogenic substrates will facilitate the discovery of high-quality SIRT inhibitors. We here report 16 new fluorogenic peptide substrates (P1–P16) designed with structurally diverse tetrapeptides and acyl modifications. Tests of P1–P16 against SIRT isoforms identified several sensitive substrates for SIRT1, SIRT2, SIRT3 and SIRT5, which manifested lower KM values and higher catalytic efficiency, and particularly had less signal interference in inhibitor screening compared with our previously reported internally quenched fluorescent substrates. Co-crystallization of sensitive substrates P13 and P15 with SIRT5 revealed an unexpected binding mode, involving interactions with residues from active site bordering surfaces, different from that observed for other peptides derived from natural protein substrates. By using SIRT5 sensitive substrates, we found that TW-37, a Bcl-2 inhibitor, displayed low micromolar inhibition to SIRT5, which was further validated by isothermal titration calorimetry analyses, offering a new point to develop dual-action SIRT5/Bcl-2 inhibitors against cancers. This work provides assay platform and structural basis for developing new substrates and inhibitors targeting human SIRTs.
中文翻译:

瑟土因脱酰基酶抑制剂发现的敏感荧光底物
Sirtuins(SIRT)是依赖NAD +的赖氨酸脱酰基酶,调节许多重要的生物学过程,例如代谢和应激反应。SIRT抑制剂可能会对抗SIRT驱动的人类疾病。基于荧光底物的高效检测平台的开发将有助于发现高质量的SIRT抑制剂。我们在这里报告了16种新的荧光肽底物(P1 – P16),其设计具有结构上不同的四肽和酰基修饰。针对SIRT同工型的P1 - P16测试确定了SIRT1,SIRT2,SIRT3和SIRT5的几种敏感底物,其K M较低与我们先前报道的内部淬灭荧光底物相比,它具有更高的催化效率和更高的催化效率,特别是在抑制剂筛选中信号干扰更小。敏感基板P13和P15的共结晶SIRT5的结合揭示了一种意想不到的结合模式,涉及与活性位点边界表面残基的相互作用,这与天然蛋白底物衍生的其他肽所观察到的不同。通过使用对SIRT5敏感的底物,我们发现Bcl-2抑制剂TW-37对SIRT5表现出较低的微摩尔抑制作用,这通过等温滴定热分析进一步验证,为开发双重作用SIRT5 / Bcl-2提供了新的观点抗癌抑制剂。这项工作为开发针对人类SIRT的新底物和抑制剂提供了测定平台和结构基础。











































 京公网安备 11010802027423号
京公网安备 11010802027423号