Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.bmc.2020.115407 Akihiro Ohkubo , Kousuke Muto , Rintaro Watanabe , Shuhei Nishizawa , Shugo Hisamatsu , Takashi Kanamori
|
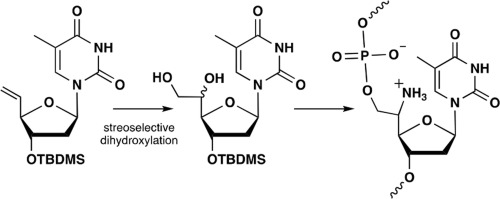
In this study, we designed 5′-amino-5′-deoxy-5′-hydroxymethylthymidine as a new oligonucleotide modification with an amino group directly attached to the 5′-carbon atom. We successfully synthesized two isomers of 5′-amino-5′-deoxy-5′-hydroxymethylthymidine via dihydroxylation of the 5′-vinyl group incorporated into 5′-deoxy-5′-C-methenylthymidine derivartive. Moreover, it was found that the nuclease resistance, binding selectivity to single-stranded RNA, and triplex-forming ability of an oligonucleotide containing RT residues of the new compound were higher than those of the unmodified oligonucleotide.
中文翻译:

5'-氨基-5'-脱氧-5'-羟甲基胸苷残基修饰寡核苷酸的化学合成及性质
在这项研究中,我们设计了5'-氨基-5'-脱氧-5'-羟甲基胸苷作为新的寡核苷酸修饰,其氨基直接连接到5'-碳原子上。我们通过掺入5'-脱氧-5' - C-亚甲基胸苷衍生物的5'-乙烯基的二羟基化成功合成了5'-氨基-5'-脱氧-5'-羟甲基胸苷的两种异构体。此外,发现含有新化合物的R T残基的寡核苷酸的核酸酶抗性,对单链RNA的结合选择性和形成三链体的能力高于未修饰的寡核苷酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号