Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.bmc.2020.115406 Mohammed I. El-Gamal , Seyed-Omar Zaraei , Paul A. Foster , Hanan S. Anbar , Randa El-Gamal , Raafat El-Awady , Barry V.L. Potter
|
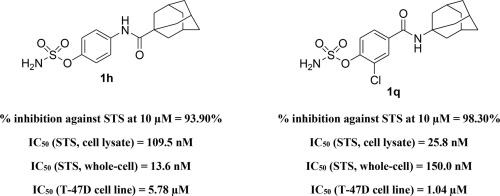
Steroid sulfatase (STS) has recently emerged as a drug target for management of hormone-dependent malignancies. In the present study, a new series of twenty-one aryl amido-linked sulfamate derivatives 1a-u was designed and synthesized, based upon a cyclohexyl lead compound. All members were evaluated as STS inhibitors in a cell-free assay. Adamantyl derivatives 1h and 1p-r were the most active with more than 90% inhibition at 10 µM concentration and, for those with the greatest inhibitory activity, IC50 values were determined. These compounds exhibited STS inhibition within the range of ca 25-110 nM. Amongst them, compound 1q possessing a o-chlorobenzene sulfamate moiety exhibited the most potent STS inhibitory activity with an IC50 of 26 nM. Furthermore, to assure capability to pass through the cell lipid bilayer, compounds with low IC50 values were tested against STS activity in JEG-3 whole-cell assays. Consequently, 1h and 1q demonstrated IC50 values of ca 14 and 150 nM, respectively. Thus, compound 1h is 31 times more potent than the corresponding cyclohexyl lead (IC50 value = 421 nM in a JEG-3 whole-cell assay). Furthermore, the most potent STS inhibitors (1h and 1p-r) were evaluated for their antiproliferative activity against the estrogen-dependent breast cancer cell line T-47D. They showed promising activity with single digit micromolar IC50 values (ca 1-6 µM) and their potency against T-47D cells was comparable to that against STS enzyme. In conclusion, this new class of adamantyl-containing aryl sulfamate inhibitor has potential for further development against hormone-dependent tumours.
中文翻译:

氨基磺酸氨基磺酸酯衍生物的新系列:设计,合成和生物学评估
甾族硫酸酯酶(STS)最近已成为治疗激素依赖性恶性肿瘤的药物靶标。在本研究中,基于环己基铅化合物,设计并合成了一系列新的二十一个芳基酰胺基连接的氨基磺酸酯衍生物1a-u。在无细胞试验中将所有成员评估为STS抑制剂。金刚烷衍生物1h和1p-r在10 µM浓度下活性最高,抑制率超过90%,对于那些抑制活性最大的化合物,测定IC 50值。这些化合物在约25-110nM的范围内表现出STS抑制。其中,化合物1q具有o-氯苯磺酸氨基酯部分表现出最有效的STS抑制活性,IC 50为26 nM。此外,为了确保能够穿过细胞脂质双层,在JEG-3全细胞试验中针对STS活性测试了具有低IC 50值的化合物。因此,1h和1q的IC 50值分别约为14 nM和150 nM。因此,化合物1h的效力是相应的环己基铅的31倍(在JEG-3全细胞试验中,IC 50值= 421 nM)。此外,最有效的STS抑制剂(1h和1p-r评估了其对雌激素依赖性乳腺癌细胞系T-47D的抗增殖活性。他们表现出令人鼓舞的活性,其微摩尔IC 50值为一位(约1-6 µM),它们对T-47D细胞的效力与对STS酶的效力相当。总之,这种新型的含金刚烷基芳基氨基磺酸盐抑制剂具有抗激素依赖性肿瘤的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号