Applied Catalysis A: General ( IF 4.7 ) Pub Date : 2020-03-03 , DOI: 10.1016/j.apcata.2020.117505 Preeyaporn Poldorn , Yutthana Wongnongwa , Supawadee Namuangruk , Nawee Kungwan , Vladimir B. Golovko , Burapat Inceesungvorn , Siriporn Jungsuttiwong
|
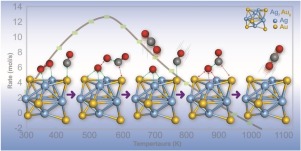
We report an advanced configurational sampling method that uses density functional theory (DFT) to design a highly active catalyst for conversion of CO into less-harmful products, under ambient conditions. The reaction pathway for CO oxidation by O2 on ultra-small 13-Atom bimetallic Ag7Au6 cluster has two possible mechanisms, namely, stepwise adsorption and co-adsorption. The rate-determining step involving with CO-O association via a co-adsorption process shows a significantly small barrier of 0.21 eV. Furthermore, microkinetic simulation results suggest that CO oxidation rates and the optimal temperature for CO oxidation exhibit both greater performances for the co-adsorption pathway, compared to that for a stepwise-adsorption mechanism. Our new proposed mechanism suggests that the bimetallic Ag7Au6 catalyst is active for CO oxidation at room temperatures. Thus, it has potential application as a highly-active catalyst for conversion of carbon monoxide into less toxic CO2.
中文翻译:

超小型13原子双金属Ag 7 Au 6团簇上O 2催化CO氧化CO的理论机理研究
我们报告了一种先进的配置采样方法,该方法使用密度泛函理论(DFT)设计了一种高活性催化剂,用于在环境条件下将CO转化为危害较小的产品。O 2在超小13原子双金属Ag 7 Au 6簇上被O 2氧化CO的反应途径有两种可能的机理,即逐步吸附和共吸附。通过CO-O关联进行的速率确定步骤共吸附过程显示出0.21 eV的很小的势垒。此外,微动力学模拟结果表明,与逐步吸附机理相比,CO氧化速率和最佳氧化温度都对共吸附途径表现出更高的性能。我们提出的新机理表明,双金属Ag 7 Au 6催化剂在室温下对CO氧化具有活性。因此,它具有作为高活性催化剂用于将一氧化碳转化为毒性较小的CO 2的潜力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号