当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assessment of Thermochemical Data of γ-Butyrolactone from Experimental and Computational Studies
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.jced.9b01135 Arturo Ximello-Hernández 1 , Vera L. S. Freitas 1 , Maria D. M. C. Ribeiro da Silva 1
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.jced.9b01135 Arturo Ximello-Hernández 1 , Vera L. S. Freitas 1 , Maria D. M. C. Ribeiro da Silva 1
Affiliation
|
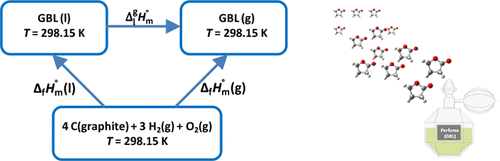
Relevant thermochemical data of γ-butyrolactone (GBL), a compound of considerable industrial and environmental significance, obtained from experimental and computational studies are reported in this work. The standard (p° = 0.1 MPa) molar enthalpy of formation, at T = 298.15 K, in the gaseous phase, was determined from the enthalpy of combustion and vaporization, obtained by static bomb calorimetry in oxygen and by Calvet microcalorimetry, respectively. The enthalpy of formation in the gaseous phase was also determined from G3 calculations. The excellent agreement between the experimental and computational thermochemical data provided a critical literature review of GBL and the proposal of new reference values for this lactone.
中文翻译:

通过实验和计算研究评估γ-丁内酯的热化学数据
这项工作报告了从实验和计算研究中获得的具有重要工业和环境意义的化合物γ-丁内酯(GBL)的相关热化学数据。在气相中,在T = 298.15 K时,由燃烧和汽化焓确定标准(p °= 0.1 MPa)摩尔形成焓,该燃烧和汽化焓分别通过在氧气中的静态炸弹量热法和Calvet微量量热法获得。气相中的生成焓也由G3计算确定。实验和计算热化学数据之间的极好的一致性为GBL提供了重要的文献综述,并为该内酯提供了新的参考值。
更新日期:2020-04-24
中文翻译:

通过实验和计算研究评估γ-丁内酯的热化学数据
这项工作报告了从实验和计算研究中获得的具有重要工业和环境意义的化合物γ-丁内酯(GBL)的相关热化学数据。在气相中,在T = 298.15 K时,由燃烧和汽化焓确定标准(p °= 0.1 MPa)摩尔形成焓,该燃烧和汽化焓分别通过在氧气中的静态炸弹量热法和Calvet微量量热法获得。气相中的生成焓也由G3计算确定。实验和计算热化学数据之间的极好的一致性为GBL提供了重要的文献综述,并为该内酯提供了新的参考值。











































 京公网安备 11010802027423号
京公网安备 11010802027423号