当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Edaravone in Four Mixed Solvents at 273.15–313.15 K and Correlation of Jouyban–Acree and CNIBS/R–K Models
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-02 , DOI: 10.1021/acs.jced.9b00881 Xiaoyu Wu 1 , Xianfang Yin 2 , Tian Tang 2 , Huilin Zheng 2 , Wenjie Xu 2 , Zhipeng Lin 2 , Xianlang Chen 2 , Rongrong Li 2 , Jia Zhao 3 , Deman Han 2
Journal of Chemical & Engineering Data ( IF 2.0 ) Pub Date : 2020-03-02 , DOI: 10.1021/acs.jced.9b00881 Xiaoyu Wu 1 , Xianfang Yin 2 , Tian Tang 2 , Huilin Zheng 2 , Wenjie Xu 2 , Zhipeng Lin 2 , Xianlang Chen 2 , Rongrong Li 2 , Jia Zhao 3 , Deman Han 2
Affiliation
|
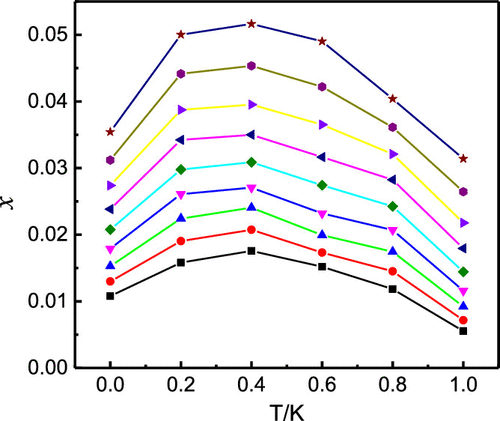
The solubility of edaravone in four mixed solvents including (ethyl acetate + n-propanol), (acetonitrile + methanol), (acetonitrile + ethanol), and (acetonitrile + n-propanol) was determined by using the isothermal saturation method at temperatures ranging from (273.15 to 313.15) K under 101.3 kPa. At a certain composition in the four mixed solvents, the solubility data of edaravone increased with the rising temperature. At a certain temperature, the solubility data decreased monotonically with the increasing mass fraction of acetonitrile in the mixture of (acetonitrile + n-propanol). Whereas in (ethyl acetate + n-propanol), (acetonitrile + methanol), and (acetonitrile + ethanol), it increased first and then decreased with the increasing mass fraction of ethyl acetate or acetonitrile. The composition dependence of solubility has a maximum at around wethyl acetate = 0.2 in (ethyl acetate + n-propanol) or wacetonitrile = 0.2 in (acetonitrile + ethanol) and wacetonitrile = 0.4 for mixtures of (acetonitrile + methanol). The results obtained in the selected four mixtures were correlated by the Jouyban–Acree model and CNIBS/R–K model. The largest relative average deviation and root-mean-square deviation values were found in (acetonitrile + methanol) calculated by the Jouyban–Acree model and were no more than 4.71 × 10–2 and 22.32 × 10–4, respectively. Statistical analysis of experimental and calculated values shows that the residual of CNIBS/R–K model is smaller, and the linear correlation coefficient is closer to 1. Therefore, the CNIBS/R–K model is more suitable to correlate the solubility values of edaravone in the four mixed solvents including (ethyl acetate + n-propanol), (acetonitrile + methanol), (acetonitrile + ethanol), and (acetonitrile + n-propanol). The experimental data and model parameters obtained will play an important role in the preparation and purification of edaravone.
中文翻译:

依达拉奉在273.15–313.15 K的四种混合溶剂中的溶解度以及Jouyban–Acree模型和CNIBS / R–K模型的相关性
依达拉奉在以下温度下使用等温饱和法测定在(乙酸乙酯+正丙醇),(乙腈+甲醇),(乙腈+乙醇)和(乙腈+正丙醇)四种混合溶剂中的溶解度(273.15至313.15)K在101.3 kPa以下。在四种混合溶剂中一定的组成下,依达拉奉的溶解度数据随温度升高而增加。在一定温度下,溶解度数据随(乙腈+正丙醇)混合物中乙腈质量分数的增加而单调降低。而在(乙酸乙酯+ n-丙醇),(乙腈+甲醇)和(乙腈+乙醇),随着乙酸乙酯或乙腈的质量分数增加,其先升高后降低。溶解度的成分相关性最大,大约在w乙酸乙酯= 0.2英寸(乙酸乙酯+正丙醇)或w乙腈= 0.2英寸(乙腈+乙醇)和w乙腈附近对于(乙腈+甲醇)混合物,= 0.4。通过Jouyban–Acree模型和CNIBS / R–K模型将在选定的四种混合物中获得的结果进行关联。在通过Jouyban-Acree模型计算的(乙腈+甲醇)中发现最大的相对平均偏差和均方根偏差值分别不超过4.71×10 –2和22.32×10 –4。实验值和计算值的统计分析表明,CNIBS / R–K模型的残差较小,线性相关系数更接近1。因此,CNIBS / R–K模型更适合于关联依达拉奉的溶解度值在四种混合溶剂中,包括(乙酸乙酯+ n-丙醇),(乙腈+甲醇),(乙腈+乙醇)和(乙腈+正丙醇)。获得的实验数据和模型参数将在依达拉奉的制备和纯化中起重要作用。
更新日期:2020-04-24
中文翻译:

依达拉奉在273.15–313.15 K的四种混合溶剂中的溶解度以及Jouyban–Acree模型和CNIBS / R–K模型的相关性
依达拉奉在以下温度下使用等温饱和法测定在(乙酸乙酯+正丙醇),(乙腈+甲醇),(乙腈+乙醇)和(乙腈+正丙醇)四种混合溶剂中的溶解度(273.15至313.15)K在101.3 kPa以下。在四种混合溶剂中一定的组成下,依达拉奉的溶解度数据随温度升高而增加。在一定温度下,溶解度数据随(乙腈+正丙醇)混合物中乙腈质量分数的增加而单调降低。而在(乙酸乙酯+ n-丙醇),(乙腈+甲醇)和(乙腈+乙醇),随着乙酸乙酯或乙腈的质量分数增加,其先升高后降低。溶解度的成分相关性最大,大约在w乙酸乙酯= 0.2英寸(乙酸乙酯+正丙醇)或w乙腈= 0.2英寸(乙腈+乙醇)和w乙腈附近对于(乙腈+甲醇)混合物,= 0.4。通过Jouyban–Acree模型和CNIBS / R–K模型将在选定的四种混合物中获得的结果进行关联。在通过Jouyban-Acree模型计算的(乙腈+甲醇)中发现最大的相对平均偏差和均方根偏差值分别不超过4.71×10 –2和22.32×10 –4。实验值和计算值的统计分析表明,CNIBS / R–K模型的残差较小,线性相关系数更接近1。因此,CNIBS / R–K模型更适合于关联依达拉奉的溶解度值在四种混合溶剂中,包括(乙酸乙酯+ n-丙醇),(乙腈+甲醇),(乙腈+乙醇)和(乙腈+正丙醇)。获得的实验数据和模型参数将在依达拉奉的制备和纯化中起重要作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号