当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Investigation into the Oxidation of Guanine to Form Imidazolone (Iz) and Related Degradation Products.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.chemrestox.0c00039 Sebastien P Hebert 1 , H Bernhard Schlegel 1
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-03-20 , DOI: 10.1021/acs.chemrestox.0c00039 Sebastien P Hebert 1 , H Bernhard Schlegel 1
Affiliation
|
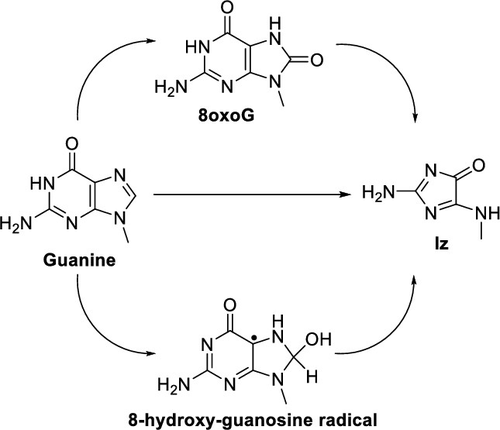
Imidazolone (Iz) is one of the many products resulting from oxidative damage to DNA. Three pathways for the formation of Iz and related degradation products have been studied by density functional theory using the ωB97XD functional with the 6-31+G(d,p) basis set and SMD implicit water solvation plus a small number of explicit water molecules positioned to help stabilize charged species and facilitate reaction steps. The first pathway starts with guanine radical and the addition of superoxide at C5. Endoperoxide formation was calculated to have slightly lower barriers than diol formation. The next steps are pyrimidine ring opening and decarboxylation. Ring migration then proceeds via an acyclic intermediate rather than a bicyclic intermediate and is followed by formamide loss to yield Iz. The second pathway starts with 8oxoG and proceeds via C5 superoxide addition and diol formation to a relatively stable intermediate, oxidized guanidinohydantoin (Ghox). The barriers for hydroxide ion addition to Ghox are much lower than for water addition and should yield more Iz and parabanic acid at higher pH. The third pathway starts with 8-hydroxy guanine radical formed by hydroxyl radical addition to C8 of guanine or water addition to C8 of guanine radical. Superoxide addition at C5 is followed by diol formation, ring opening and decarboxylation similar to pathways 1 and 2, subsequently leading to Iz formation. The calculated pathways are in good agreement with experimental observations.
中文翻译:

鸟嘌呤氧化形成咪唑酮(Iz)及其相关降解产物的计算研究。
咪唑啉酮(Iz)是DNA氧化损伤所产生的众多产品之一。利用密度泛函理论,使用具有6-31 + G(d,p)基组的ωB97XD官能团和SMD隐性水溶剂化以及少量的显性水分子,通过密度泛函理论研究了形成Iz和相关降解产物的三个途径帮助稳定带电物质并促进反应步骤。第一条途径始于鸟嘌呤自由基和在C5处添加超氧化物。计算出过氧化物的形成具有比二醇形成略低的势垒。下一步是嘧啶开环和脱羧。环迁移然后通过无环中间体而不是双环中间体进行,然后甲酰胺损失,得到Iz。第二个途径从8oxoG开始,并通过C5超氧化物加成和二醇的形成进入相对稳定的中间体,氧化胍基乙内酰脲(Ghox)。向Ghox中添加氢氧根离子的阻隔性比向水中添加时的阻隔性低得多,并且在较高的pH值下应能产生更多的Iz和对羟基苯甲酸。第三种途径开始于8-羟基鸟嘌呤基团,该8-羟基鸟嘌呤基团是由向鸟嘌呤的C8添加羟基自由基或向鸟嘌呤的C8添加水而形成的。在C5加入超氧化物之后,类似于途径1和2,形成二醇,开环和脱羧,随后导致Iz的形成。计算出的途径与实验观察结果非常吻合。向Ghox中添加氢氧根离子的阻隔性比向水中添加时的阻隔性低得多,并且在更高的pH值下应能产生更多的Iz和旁苯甲酸。第三种途径开始于8-羟基鸟嘌呤基团,该8-羟基鸟嘌呤基团是由向鸟嘌呤的C8加羟基或向鸟嘌呤的C8加水而形成的。在C5加入超氧化物之后,类似于途径1和2,形成二醇,开环和脱羧,随后导致Iz的形成。计算出的途径与实验观察结果非常吻合。向Ghox中添加氢氧根离子的阻隔性比向水中添加时的阻隔性低得多,并且在较高的pH值下应能产生更多的Iz和旁苯甲酸。第三种途径开始于8-羟基鸟嘌呤基团,该8-羟基鸟嘌呤基团是由向鸟嘌呤的C8添加羟基自由基或向鸟嘌呤的C8添加水而形成的。在C5加入超氧化物之后,类似于途径1和2,形成二醇,开环和脱羧,随后导致Iz的形成。计算出的途径与实验观察结果非常吻合。类似于途径1和2的开环和脱羧,随后导致Iz的形成。计算出的途径与实验观察结果非常吻合。类似于途径1和2的开环和脱羧,随后导致Iz的形成。计算出的途径与实验观察结果非常吻合。
更新日期:2020-04-23
中文翻译:

鸟嘌呤氧化形成咪唑酮(Iz)及其相关降解产物的计算研究。
咪唑啉酮(Iz)是DNA氧化损伤所产生的众多产品之一。利用密度泛函理论,使用具有6-31 + G(d,p)基组的ωB97XD官能团和SMD隐性水溶剂化以及少量的显性水分子,通过密度泛函理论研究了形成Iz和相关降解产物的三个途径帮助稳定带电物质并促进反应步骤。第一条途径始于鸟嘌呤自由基和在C5处添加超氧化物。计算出过氧化物的形成具有比二醇形成略低的势垒。下一步是嘧啶开环和脱羧。环迁移然后通过无环中间体而不是双环中间体进行,然后甲酰胺损失,得到Iz。第二个途径从8oxoG开始,并通过C5超氧化物加成和二醇的形成进入相对稳定的中间体,氧化胍基乙内酰脲(Ghox)。向Ghox中添加氢氧根离子的阻隔性比向水中添加时的阻隔性低得多,并且在较高的pH值下应能产生更多的Iz和对羟基苯甲酸。第三种途径开始于8-羟基鸟嘌呤基团,该8-羟基鸟嘌呤基团是由向鸟嘌呤的C8添加羟基自由基或向鸟嘌呤的C8添加水而形成的。在C5加入超氧化物之后,类似于途径1和2,形成二醇,开环和脱羧,随后导致Iz的形成。计算出的途径与实验观察结果非常吻合。向Ghox中添加氢氧根离子的阻隔性比向水中添加时的阻隔性低得多,并且在更高的pH值下应能产生更多的Iz和旁苯甲酸。第三种途径开始于8-羟基鸟嘌呤基团,该8-羟基鸟嘌呤基团是由向鸟嘌呤的C8加羟基或向鸟嘌呤的C8加水而形成的。在C5加入超氧化物之后,类似于途径1和2,形成二醇,开环和脱羧,随后导致Iz的形成。计算出的途径与实验观察结果非常吻合。向Ghox中添加氢氧根离子的阻隔性比向水中添加时的阻隔性低得多,并且在较高的pH值下应能产生更多的Iz和旁苯甲酸。第三种途径开始于8-羟基鸟嘌呤基团,该8-羟基鸟嘌呤基团是由向鸟嘌呤的C8添加羟基自由基或向鸟嘌呤的C8添加水而形成的。在C5加入超氧化物之后,类似于途径1和2,形成二醇,开环和脱羧,随后导致Iz的形成。计算出的途径与实验观察结果非常吻合。类似于途径1和2的开环和脱羧,随后导致Iz的形成。计算出的途径与实验观察结果非常吻合。类似于途径1和2的开环和脱羧,随后导致Iz的形成。计算出的途径与实验观察结果非常吻合。











































 京公网安备 11010802027423号
京公网安备 11010802027423号