当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exception That Proves the Rule: Investigation of Privileged Stereochemistry in Designing Dopamine D3R Bitopic Agonists
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-02-28 , DOI: 10.1021/acsmedchemlett.9b00660 Francisco O Battiti 1 , Amy Hauck Newman 1 , Alessandro Bonifazi 1
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-02-28 , DOI: 10.1021/acsmedchemlett.9b00660 Francisco O Battiti 1 , Amy Hauck Newman 1 , Alessandro Bonifazi 1
Affiliation
|
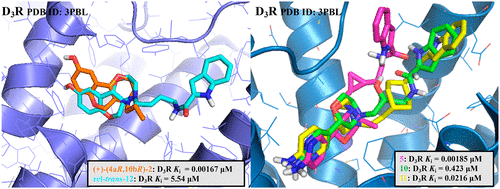
In this study, starting from our selective D3R agonist FOB02-04A (5), we investigated the chemical space around the linker portion of the molecule via insertion of a hydroxyl substituent and ring-expansion of the trans-cyclopropyl moiety into a trans-cyclohexyl scaffold. Moreover, to further elucidate the importance of the primary pharmacophore stereochemistry in the design of bitopic ligands, we investigated the chiral requirements of (+)-PD128907 ((+)-(4aR,10bR)-2)) by synthesizing and resolving bitopic analogues in all the cis and trans combinations of its 9-methoxy-3,4,4a,10b-tetrahydro-2H,5H-chromeno[4,3-b][1,4] oxazine scaffold. Despite the lack of success in obtaining new analogues with improved biological profiles, in comparison to our current leads, a “negative” result due to a poor or simply not improved biological profile is fundamental toward better understanding chemical space and optimal stereochemistry for target recognition. Herein, we identified essential structural information to understand the differences between orthosteric and bitopic ligand–receptor binding interactions, discriminate D3R active and inactive states, and assist multitarget receptor recognition. Exploring stereochemical complexity and developing extended D3R SAR from this new library complements previously described SAR and inspires future structural and computational biology investigation. Moreover, the expansion of chemical space characterization for D3R agonism may be utilized in machine learning and artificial intelligence (AI)-based drug design, in the future.
中文翻译:

证明规则的例外:在设计多巴胺 D3R 双位激动剂时研究特权立体化学
在这项研究中,从我们的选择性 D 3 R 激动剂FOB02-04A ( 5 ) 开始,我们通过插入羟基取代基和反式环丙基部分环扩展成反式,研究了分子接头部分周围的化学空间。-环己基支架。此外,为了进一步阐明主要药效团立体化学在双位配体设计中的重要性,我们通过合成和解析研究了(+)-PD128907 ( (+)-(4a R ,10b R )-2) )的手性要求所有顺式和反式中的双位类似物其-9-甲氧基-3,4,4a,10b-四氢-2-组合ħ,5 ħ -chromeno [4,3- b ] [1,4]恶嗪骨架。尽管在获得具有改进生物特征的新类似物方面缺乏成功,但与我们目前的先导相比,由于生物特征较差或根本没有改进而导致的“负面”结果对于更好地理解化学空间和用于目标识别的最佳立体化学至关重要。在此,我们确定了必要的结构信息,以了解正构和双位配体-受体结合相互作用之间的差异,区分 D 3 R 活性和非活性状态,并协助多靶点受体识别。探索立体化学的复杂性并开发扩展的 D 3来自这个新库的 R SAR 补充了之前描述的 SAR,并激发了未来的结构和计算生物学研究。此外,D 3 R 激动的化学空间表征的扩展可能在未来用于机器学习和基于人工智能 (AI) 的药物设计。
更新日期:2020-02-28
中文翻译:

证明规则的例外:在设计多巴胺 D3R 双位激动剂时研究特权立体化学
在这项研究中,从我们的选择性 D 3 R 激动剂FOB02-04A ( 5 ) 开始,我们通过插入羟基取代基和反式环丙基部分环扩展成反式,研究了分子接头部分周围的化学空间。-环己基支架。此外,为了进一步阐明主要药效团立体化学在双位配体设计中的重要性,我们通过合成和解析研究了(+)-PD128907 ( (+)-(4a R ,10b R )-2) )的手性要求所有顺式和反式中的双位类似物其-9-甲氧基-3,4,4a,10b-四氢-2-组合ħ,5 ħ -chromeno [4,3- b ] [1,4]恶嗪骨架。尽管在获得具有改进生物特征的新类似物方面缺乏成功,但与我们目前的先导相比,由于生物特征较差或根本没有改进而导致的“负面”结果对于更好地理解化学空间和用于目标识别的最佳立体化学至关重要。在此,我们确定了必要的结构信息,以了解正构和双位配体-受体结合相互作用之间的差异,区分 D 3 R 活性和非活性状态,并协助多靶点受体识别。探索立体化学的复杂性并开发扩展的 D 3来自这个新库的 R SAR 补充了之前描述的 SAR,并激发了未来的结构和计算生物学研究。此外,D 3 R 激动的化学空间表征的扩展可能在未来用于机器学习和基于人工智能 (AI) 的药物设计。











































 京公网安备 11010802027423号
京公网安备 11010802027423号