当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, Synthesis, and X-ray Studies of Potent HIV-1 Protease Inhibitors with P2-Carboxamide Functionalities
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-03 , DOI: 10.1021/acsmedchemlett.9b00670 Arun K Ghosh 1, 1 , Alessandro Grillo 1, 1 , Jakka Raghavaiah 1 , Satish Kovela 1 , Megan E Johnson 1 , Daniel W Kneller 2 , Yuan-Fang Wang 2 , Shin-Ichiro Hattori 3 , Nobuyo Higashi-Kuwata 3 , Irene T Weber 2 , Hiroaki Mitsuya 3, 4, 5
ACS Medicinal Chemistry Letters ( IF 3.5 ) Pub Date : 2020-03-03 , DOI: 10.1021/acsmedchemlett.9b00670 Arun K Ghosh 1, 1 , Alessandro Grillo 1, 1 , Jakka Raghavaiah 1 , Satish Kovela 1 , Megan E Johnson 1 , Daniel W Kneller 2 , Yuan-Fang Wang 2 , Shin-Ichiro Hattori 3 , Nobuyo Higashi-Kuwata 3 , Irene T Weber 2 , Hiroaki Mitsuya 3, 4, 5
Affiliation
|
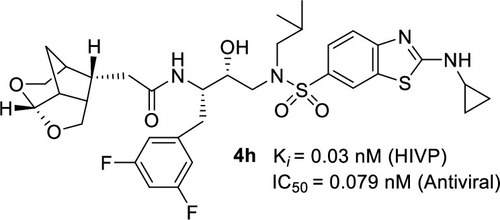
The design, synthesis, biological evaluation, and X-ray structural studies are reported for a series of highly potent HIV-1 protease inhibitors. The inhibitors incorporated stereochemically defined amide-based bicyclic and tricyclic ether derivatives as the P2 ligands with (R)-hydroxyethylaminesulfonamide transition-state isosteres. A number of inhibitors showed excellent HIV-1 protease inhibitory and antiviral activity; however, ligand combination is critical for potency. Inhibitor 4h with a difluorophenylmethyl as the P1 ligand, crown-THF-derived acetamide as the P2 ligand, and a cyclopropylaminobenzothiazole P2′-ligand displayed very potent antiviral activity and maintained excellent antiviral activity against selected multidrug-resistant HIV-1 variants. A high resolution X-ray structure of inhibitor 4h-bound HIV-1 protease provided molecular insight into the binding properties of the new inhibitor.
中文翻译:

具有 P2-甲酰胺功能的强效 HIV-1 蛋白酶抑制剂的设计、合成和 X 射线研究
报告了一系列高效 HIV-1 蛋白酶抑制剂的设计、合成、生物学评估和 X 射线结构研究。抑制剂将立体化学定义的基于酰胺的双环和三环醚衍生物作为 P2 配体与 ( R )-羟乙基胺磺酰胺过渡态等排体结合。许多抑制剂表现出优异的 HIV-1 蛋白酶抑制和抗病毒活性;然而,配体组合对效力至关重要。抑制剂4h以二氟苯甲基作为 P1 配体,冠-THF 衍生的乙酰胺作为 P2 配体,以及环丙基氨基苯并噻唑 P2'-配体显示出非常有效的抗病毒活性,并对选定的多重耐药 HIV-1 变体保持优异的抗病毒活性。抑制剂4h结合的 HIV-1 蛋白酶的高分辨率 X 射线结构提供了对新抑制剂结合特性的分子洞察。
更新日期:2020-03-03
中文翻译:

具有 P2-甲酰胺功能的强效 HIV-1 蛋白酶抑制剂的设计、合成和 X 射线研究
报告了一系列高效 HIV-1 蛋白酶抑制剂的设计、合成、生物学评估和 X 射线结构研究。抑制剂将立体化学定义的基于酰胺的双环和三环醚衍生物作为 P2 配体与 ( R )-羟乙基胺磺酰胺过渡态等排体结合。许多抑制剂表现出优异的 HIV-1 蛋白酶抑制和抗病毒活性;然而,配体组合对效力至关重要。抑制剂4h以二氟苯甲基作为 P1 配体,冠-THF 衍生的乙酰胺作为 P2 配体,以及环丙基氨基苯并噻唑 P2'-配体显示出非常有效的抗病毒活性,并对选定的多重耐药 HIV-1 变体保持优异的抗病毒活性。抑制剂4h结合的 HIV-1 蛋白酶的高分辨率 X 射线结构提供了对新抑制剂结合特性的分子洞察。











































 京公网安备 11010802027423号
京公网安备 11010802027423号