当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ancistrosecolines A-F, Unprecedented seco-Naphthylisoquinoline Alkaloids from the Roots of Ancistrocladus abbreviatus, with Apoptosis-Inducing Potential against HeLa Cancer Cells.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.jnatprod.9b01168 Shaimaa Fayez 1, 2 , Torsten Bruhn 3 , Doris Feineis 1 , Laurent Aké Assi 4 , Suresh Awale 5 , Gerhard Bringmann 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-03 , DOI: 10.1021/acs.jnatprod.9b01168 Shaimaa Fayez 1, 2 , Torsten Bruhn 3 , Doris Feineis 1 , Laurent Aké Assi 4 , Suresh Awale 5 , Gerhard Bringmann 1
Affiliation
|
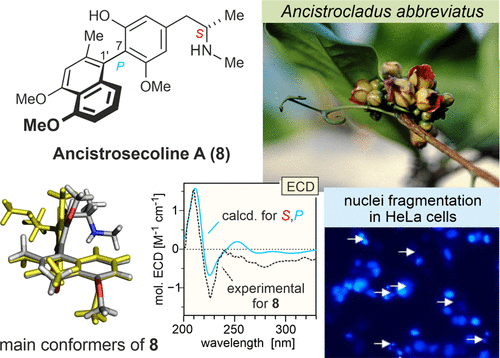
Ancistrosecolines A-F (8-13) are the first seco-type naphthylisoquinoline alkaloids discovered in Nature. In all these novel compounds, the tetrahydroisoquinoline ring is cleaved, with loss of C-1. They were isolated from the root bark of Ancistrocladus abbreviatus (Ancistrocladaceae), along with 1-nor-8-O-demethylancistrobrevine H (14), which is the first naturally occurring naphthylisoquinoline lacking the otherwise generally present methyl group at C-1. The stereostructures of the new alkaloids were established by HRESIMS, 1D and 2D NMR, oxidative degradation, and experimental and quantum-chemical ECD investigations. Ancistrosecolines A-F (8-13) and 1-nor-8-O-demethylancistrobrevine H (14) are typical Ancistrocladaceae-type metabolites, i.e., oxygenated at C-6 and S-configured at C-3, belonging to the subclasses of 7,1'- and 7,8'-coupled alkaloids. The biaryl linkages of 8-14 are rotationally hindered due to bulky ortho-substituents next to the axes. Owing to the constitutionally unsymmetric substitution patterns on each side of the axis, this C-C single bond represents an element of chirality in 1-nor-8-O-demethylancistrobrevine H (14) and in ancistrosecolines A-D (8-11). In ancistrosecolines E (12) and F (13), however, the likewise rotationally hindered biaryl axes do not constitute chiral elements, due to a symmetric substitution pattern, with its identical two methoxy functions at C-6 and C-8 in the phenyl subunit. And these two methoxy groups are, for the first time, not constitutionally heterotopic, but diastereotopic to each other. Ancistrosecoline D (11) exhibits strong cytotoxicity against HeLa cervical cancer cells. As visualized by Hoechst nuclei staining and by real-time imaging experiments, 11 induced massive nuclei fragmentation in HeLa cells, leading to apoptotic cell death.
中文翻译:

Ancistrosecolines AF,前所未有的山茱An根的癸二萘基异喹啉生物碱,具有诱导HeLa癌细胞凋亡的潜能。
Ancistrosecolines AF(8-13)是在自然界发现的第一种山高型萘基异喹啉碱生物碱。在所有这些新化合物中,四氢异喹啉环被裂解,而C-1丢失。它们是从南美带菌Ancistrocladus abbreviatus(Ancistrocladaceae)的根皮中分离出来的,以及1-nor-8-O-demethylancistrobrevine H(14),后者是第一个天然存在的萘基异喹啉,在C-1处通常没有甲基。通过HRESIMS,1D和2D NMR,氧化降解以及实验和量子化学ECD研究,确定了新生物碱的立体结构。Ancistrosecolines AF(8-13)和1-nor-8-O-demethylancistrobrevine H(14)是典型的Ancistrocladaceae型代谢物,即在C-6处被氧化,在C-3处被S-构型,属于7个子类。 ,1'-和7,8' 耦合生物碱。由于靠近轴的体积较大的原取代基,旋转阻碍了8-14的联芳基连接。由于轴两边的结构不对称取代模式,该CC单键代表1-nor-8-O-demethylancistrobrevine H(14)和anististrosecolines AD(8-11)中的手性元素。然而,在抗油菊酯碱E(12)和F(13)中,同样的受旋转阻碍的联芳基轴由于对称的取代方式而没有构成手性元素,在苯基的C-6和C-8处具有相同的两个甲氧基官能团亚基。并且这两个甲氧基首次在结构上不是异位的,而是彼此非对映的。Ancistrosecoline D(11)对HeLa宫颈癌细胞具有很强的细胞毒性。
更新日期:2020-03-03
中文翻译:

Ancistrosecolines AF,前所未有的山茱An根的癸二萘基异喹啉生物碱,具有诱导HeLa癌细胞凋亡的潜能。
Ancistrosecolines AF(8-13)是在自然界发现的第一种山高型萘基异喹啉碱生物碱。在所有这些新化合物中,四氢异喹啉环被裂解,而C-1丢失。它们是从南美带菌Ancistrocladus abbreviatus(Ancistrocladaceae)的根皮中分离出来的,以及1-nor-8-O-demethylancistrobrevine H(14),后者是第一个天然存在的萘基异喹啉,在C-1处通常没有甲基。通过HRESIMS,1D和2D NMR,氧化降解以及实验和量子化学ECD研究,确定了新生物碱的立体结构。Ancistrosecolines AF(8-13)和1-nor-8-O-demethylancistrobrevine H(14)是典型的Ancistrocladaceae型代谢物,即在C-6处被氧化,在C-3处被S-构型,属于7个子类。 ,1'-和7,8' 耦合生物碱。由于靠近轴的体积较大的原取代基,旋转阻碍了8-14的联芳基连接。由于轴两边的结构不对称取代模式,该CC单键代表1-nor-8-O-demethylancistrobrevine H(14)和anististrosecolines AD(8-11)中的手性元素。然而,在抗油菊酯碱E(12)和F(13)中,同样的受旋转阻碍的联芳基轴由于对称的取代方式而没有构成手性元素,在苯基的C-6和C-8处具有相同的两个甲氧基官能团亚基。并且这两个甲氧基首次在结构上不是异位的,而是彼此非对映的。Ancistrosecoline D(11)对HeLa宫颈癌细胞具有很强的细胞毒性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号