当前位置:
X-MOL 学术
›
J. Nat. Prod.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Talauxins: Hybrid Phenalenone Dimers from Talaromyces stipitatus.
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-02 , DOI: 10.1021/acs.jnatprod.9b01066 Nirmal K Chaudhary 1 , Andrew Crombie 2 , Daniel Vuong 2 , Ernest Lacey 1, 2 , Andrew M Piggott 1 , Peter Karuso 1
Journal of Natural Products ( IF 3.3 ) Pub Date : 2020-03-02 , DOI: 10.1021/acs.jnatprod.9b01066 Nirmal K Chaudhary 1 , Andrew Crombie 2 , Daniel Vuong 2 , Ernest Lacey 1, 2 , Andrew M Piggott 1 , Peter Karuso 1
Affiliation
|
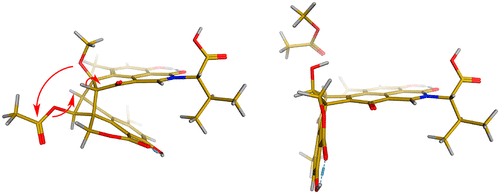
Cultivation and extraction of the fungus Talaromyces stipitatus led to the isolation of five new oxyphenalenone-amino acid hybrids, which were named talauxins E, Q, V, L, and I based on the corresponding one-letter amino acid codes, along with their putative biosynthetic precursor, duclauxin. The rapid reaction of duclauxin with amino acids to produce talauxins was demonstrated in vitro and exploited to generate a small library of natural and unnatural talauxins. Talauxin V was shown to undergo spontaneous elimination of methyl acetate to yield the corresponding neoclauxin scaffold. This process was modeled using density functional theory calculations, revealing a dramatic change in conformation resulting from the syn elimination of methyl acetate.
中文翻译:

Talauxins:来自Talaromyces stipitatus的杂酚醛酮二聚体。
栽培和提取真菌塔拉木霉(Talaromyces stipitatus)导致分离出五个新的氧苯丙烯酮-氨基酸杂种,根据相应的一个字母的氨基酸代码及其推定名称将其命名为talauxins E,Q,V,L和I生物合成前体duclauxin。在体外证明了duclauxin与氨基酸的快速反应以产生他洛辛,并被利用来生成天然和非天然他洛辛的小文库。已显示Talauxin V自发消除了乙酸甲酯,生成了相应的新clauxin支架。使用密度泛函理论计算对该过程进行建模,揭示了由于乙酸甲酯的同位消除而导致的构象的巨大变化。
更新日期:2020-03-02
中文翻译:

Talauxins:来自Talaromyces stipitatus的杂酚醛酮二聚体。
栽培和提取真菌塔拉木霉(Talaromyces stipitatus)导致分离出五个新的氧苯丙烯酮-氨基酸杂种,根据相应的一个字母的氨基酸代码及其推定名称将其命名为talauxins E,Q,V,L和I生物合成前体duclauxin。在体外证明了duclauxin与氨基酸的快速反应以产生他洛辛,并被利用来生成天然和非天然他洛辛的小文库。已显示Talauxin V自发消除了乙酸甲酯,生成了相应的新clauxin支架。使用密度泛函理论计算对该过程进行建模,揭示了由于乙酸甲酯的同位消除而导致的构象的巨大变化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号