当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Cobalt-Catalyzed Heterodimerization of Acrylates and 1,3-Dienes. A Potential Role of Cationic Cobalt(I) Intermediates.
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-23 , DOI: 10.1021/acscatal.9b05455 Montgomery Gray 1 , Michael T Hines 2 , Mahesh M Parsutkar 1 , A J Wahlstrom 2 , Nicholas A Brunelli 2 , T V RajanBabu 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-23 , DOI: 10.1021/acscatal.9b05455 Montgomery Gray 1 , Michael T Hines 2 , Mahesh M Parsutkar 1 , A J Wahlstrom 2 , Nicholas A Brunelli 2 , T V RajanBabu 1
Affiliation
|
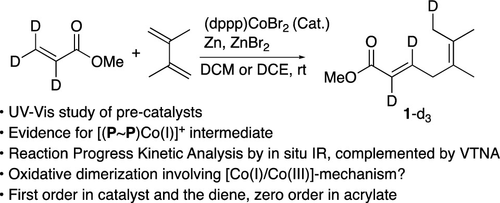
Coupling reactions of feedstock alkenes are promising, but few of these reactions are practiced industrially. Even though recent advances in the synthetic methodology have led to excellent regio- and enantioselectivies in the dimerization reactions between 1,3-dienes and acrylates, the efficiency as measured by the turnover numbers (TONs) in the catalyst has remained modest. Through a combination of reaction progress kinetic analysis (RPKA) of a prototypical dimerization reaction and characterization of isolated low-valent cobalt catalyst precursors involved, several important details of the mechanism of this reaction have emerged. (i) The prototypical reaction has an induction period that requires at least 2 h of stir time to generate the competent catalyst. (ii) Reduction of a Co(II) complex to a Co(I) complex and subsequent generation of a cationic [Co(I)]+ species are responsible for this delay. (iii) Through RPKA using in situ infrared spectroscopy, same excess experiments reveal inhibition by the product toward the end of the reaction, and no catalyst deactivation is observed as long as the diene is present in the medium. The low TON observed is most likely the result of the inherent instability of the putative cationic Co(I) species that catalyzes the reaction. (iv) Different excess experiments suggest that the reaction is first order in the diene and zero order in the acrylate. (v) Catalyst loading experiments show that the catalyst is first order. The orders in the various regents were further confirmed by variable time normalization analysis. (vi) A mechanism based on oxidative dimerization [via Co(I)/Co(III)-cycle] is proposed. Based on the results of this study, it is possible to increase the TON by a factor of 10 by conducting the reaction at an increased concentration of the starting materials, especially the diene, which seems to stabilize the catalytic species.
中文翻译:

钴催化丙烯酸酯和 1,3-二烯异二聚反应的机理。阳离子钴(I) 中间体的潜在作用。
原料烯烃的偶联反应很有前景,但这些反应很少在工业上实践。尽管合成方法的最新进展使 1,3-二烯和丙烯酸酯之间的二聚反应具有优异的区域选择性和对映选择性,但通过催化剂中的周转数 (TON) 衡量的效率仍然较低。通过结合典型二聚反应的反应进程动力学分析(RPKA)和所涉及的分离的低价钴催化剂前体的表征,揭示了该反应机理的几个重要细节。 (i) 原型反应有一个诱导期,需要至少 2 小时的搅拌时间才能产生活性催化剂。 (ii) Co(II) 络合物还原为 Co(I) 络合物以及随后生成的阳离子 [Co (I) ] +物质是造成这种延迟的原因。 (iii)通过使用原位红外光谱的RPKA,相同的过量实验揭示了反应快结束时产物的抑制,并且只要介质中存在二烯就没有观察到催化剂失活。观察到的低 TON 很可能是催化反应的假定阳离子 Co(I) 物种固有的不稳定性的结果。 (iv) 不同的过量实验表明,该反应在二烯中是一级反应,在丙烯酸酯中是零级反应。 (v)催化剂负载实验表明催化剂为一级催化剂。通过可变时间归一化分析进一步确认了各个摄政者的顺序。 (vi) 提出了一种基于氧化二聚化[通过Co(I)/Co(III)-循环]的机制。 根据这项研究的结果,通过在增加起始材料(尤其是二烯)浓度下进行反应,可以将 TON 提高 10 倍,二烯似乎可以稳定催化物质。
更新日期:2020-03-24
中文翻译:

钴催化丙烯酸酯和 1,3-二烯异二聚反应的机理。阳离子钴(I) 中间体的潜在作用。
原料烯烃的偶联反应很有前景,但这些反应很少在工业上实践。尽管合成方法的最新进展使 1,3-二烯和丙烯酸酯之间的二聚反应具有优异的区域选择性和对映选择性,但通过催化剂中的周转数 (TON) 衡量的效率仍然较低。通过结合典型二聚反应的反应进程动力学分析(RPKA)和所涉及的分离的低价钴催化剂前体的表征,揭示了该反应机理的几个重要细节。 (i) 原型反应有一个诱导期,需要至少 2 小时的搅拌时间才能产生活性催化剂。 (ii) Co(II) 络合物还原为 Co(I) 络合物以及随后生成的阳离子 [Co (I) ] +物质是造成这种延迟的原因。 (iii)通过使用原位红外光谱的RPKA,相同的过量实验揭示了反应快结束时产物的抑制,并且只要介质中存在二烯就没有观察到催化剂失活。观察到的低 TON 很可能是催化反应的假定阳离子 Co(I) 物种固有的不稳定性的结果。 (iv) 不同的过量实验表明,该反应在二烯中是一级反应,在丙烯酸酯中是零级反应。 (v)催化剂负载实验表明催化剂为一级催化剂。通过可变时间归一化分析进一步确认了各个摄政者的顺序。 (vi) 提出了一种基于氧化二聚化[通过Co(I)/Co(III)-循环]的机制。 根据这项研究的结果,通过在增加起始材料(尤其是二烯)浓度下进行反应,可以将 TON 提高 10 倍,二烯似乎可以稳定催化物质。











































 京公网安备 11010802027423号
京公网安备 11010802027423号