当前位置:
X-MOL 学术
›
Chin. J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Doublet Chirality Transfer and Reversible Helical Transition in Poly(3,5‐disubstituted phenylacetylene)s with Pyrene as a Probe Unit†
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-04-09 , DOI: 10.1002/cjoc.202000020 Sheng Wang 1 , Xuanyu Feng 1 , Jie Zhang 1 , Xinhua Wan 1
Chinese Journal of Chemistry ( IF 5.5 ) Pub Date : 2020-04-09 , DOI: 10.1002/cjoc.202000020 Sheng Wang 1 , Xuanyu Feng 1 , Jie Zhang 1 , Xinhua Wan 1
Affiliation
|
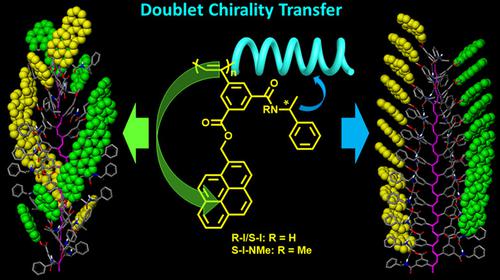
A novel doublet chirality transfer (DCT) model was demonstrated in cis poly(3,5‐disubstituted phenylacetylene)s, i.e., S‐I, R‐I, and S‐I‐NMe. The chiral message from the stereocenter of alkylamide substituent at 3‐position induced the polyene backbone to take cis‐transoid helical conformation with a predominant screw sense. And in turn the helical backbone acted as a scaffold to orient the pyrene probes, which was linked to phenyl rings through 5‐position, to array in an asymmetric manner. A combinatory analyses of 1H NMR, Raman, FTIR, UV‐vis absorption, CD, and computer simulation suggested that the main‐chain stereostructure, solvent nature, and intramolecular hydrogen bonds played important and complex roles on DCT. High cis‐structure content and intramolecular hydrogen bonds were beneficial for the realization of DCT. Reversible helix‐helix transition was observed in S‐I by changing the nature of solvents. In DMF, S‐I adopted a relatively contracted helix, where the main chain exhibited strong optical activity, but that of pyrene was weak. In contrast, a relatively stretched helix formed in CHCl3, in which the optical activity of pyrene was much larger, whereas that of the polyene backbone was the weakest. This helix‐helix transition was attributed to the intramolecular hydrogen bonds, which was confirmed by solution‐state FTIR spectra and computer calculations.
中文翻译:

以t为探针单元的聚(3,5-二取代苯基乙炔)中的双峰手性转移和可逆螺旋转变†
一种新颖的双峰手性转移(DCT)模型表现在顺式聚(3,5-二取代苯乙炔)类,即,S-I ,R-I ,和S-I-NME。来自3-位烷基酰胺取代基立体中心的手性信息诱导多烯骨架呈顺式-螺旋状螺旋构象,并具有主要的螺旋感。然后,螺旋主链充当支架来定向the探针,,探针通过5位与苯环相连,以不对称的方式排列。的组合子分析11 H NMR,拉曼光谱,FTIR,紫外可见吸收,CD和计算机模拟表明,主链立体结构,溶剂性质和分子内氢键在DCT中起着重要而复杂的作用。高顺式结构含量和分子内氢键有利于DCT的实现。通过改变溶剂的性质,在S-1中观察到可逆的螺旋-螺旋转变。在DMF中,S-1采用相对收缩的螺旋结构,其中主链表现出较强的光学活性,而pyr的光学活性较弱。相反,在CHCl 3中形成了相对拉伸的螺旋,其中of的光学活性大得多,而多烯骨架的光学活性最弱。这种螺旋-螺旋转变归因于分子内氢键,这已通过溶液状态FTIR光谱和计算机计算得到了证实。
更新日期:2020-04-09
中文翻译:

以t为探针单元的聚(3,5-二取代苯基乙炔)中的双峰手性转移和可逆螺旋转变†
一种新颖的双峰手性转移(DCT)模型表现在顺式聚(3,5-二取代苯乙炔)类,即,S-I ,R-I ,和S-I-NME。来自3-位烷基酰胺取代基立体中心的手性信息诱导多烯骨架呈顺式-螺旋状螺旋构象,并具有主要的螺旋感。然后,螺旋主链充当支架来定向the探针,,探针通过5位与苯环相连,以不对称的方式排列。的组合子分析11 H NMR,拉曼光谱,FTIR,紫外可见吸收,CD和计算机模拟表明,主链立体结构,溶剂性质和分子内氢键在DCT中起着重要而复杂的作用。高顺式结构含量和分子内氢键有利于DCT的实现。通过改变溶剂的性质,在S-1中观察到可逆的螺旋-螺旋转变。在DMF中,S-1采用相对收缩的螺旋结构,其中主链表现出较强的光学活性,而pyr的光学活性较弱。相反,在CHCl 3中形成了相对拉伸的螺旋,其中of的光学活性大得多,而多烯骨架的光学活性最弱。这种螺旋-螺旋转变归因于分子内氢键,这已通过溶液状态FTIR光谱和计算机计算得到了证实。











































 京公网安备 11010802027423号
京公网安备 11010802027423号