当前位置:
X-MOL 学术
›
New J. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fusion of thienyl into the backbone of electron acceptor in organic photovoltaic heterojunctions: a comparative study of BTPT-4F and BTPTT-4F
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020/03/02 , DOI: 10.1039/d0nj00570c Rui-Rong Bai 1, 2, 3, 4 , Cai-Rong Zhang 1, 2, 3, 4 , You-Zhi Wu 2, 3, 4, 5 , Mei-Ling Zhang 1, 2, 3, 4 , Yu-Hong Chen 1, 2, 3, 4 , Zi-Jiang Liu 3, 4, 6, 7 , Hong-Shan Chen 3, 4, 8, 9
New Journal of Chemistry ( IF 2.7 ) Pub Date : 2020/03/02 , DOI: 10.1039/d0nj00570c Rui-Rong Bai 1, 2, 3, 4 , Cai-Rong Zhang 1, 2, 3, 4 , You-Zhi Wu 2, 3, 4, 5 , Mei-Ling Zhang 1, 2, 3, 4 , Yu-Hong Chen 1, 2, 3, 4 , Zi-Jiang Liu 3, 4, 6, 7 , Hong-Shan Chen 3, 4, 8, 9
Affiliation
|
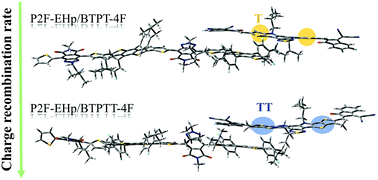
Molecular engineering of electron donors and acceptors for organic bulk heterojunction solar cells is critical to develop novel materials with improved power conversion efficiency. Here, the non-fullerene acceptors (NFAs) BTPT-4F and BTPTT-4F combined with an electron donor, P2F-EHp, were taken as a representative system to theoretically study the effects of the fusion of thienyl into the backbone of NFAs. On the basis of extensive quantum chemistry calculations, it was found that fusing thienyl into the backbone elevated the highest occupied molecular orbital (HOMO) energy, reduced the gap between the HOMO and the lowest unoccupied molecular orbital, decreased the dipole moment, enhanced the light absorbance and generated red-shifts in the absorption bands. The data for the P2F-EHp/BTPT-4F and P2F-EHp/BTPTT-4F complexes indicate that fusing thienyl into the backbone of the electron acceptor modifies the excitation character of the donor/acceptor complexes, increases the charge transfer (CT) excitation energy and the number of local excitation and CT hybridized low-lying excited states, amplifies the CT rate, reduces the exciton dissociation rate, and dramatically suppresses the charge recombination (CR) rate, leading to better performance of BTPTT-4F. However, the poorer performance of BTPT- 4F can mainly be attributed to its larger dipole moment and faster CR rate.
中文翻译:

噻吩在有机光伏异质结中融合到电子受体主链中:BTPT-4F和BTPTT-4F的比较研究
有机本体异质结太阳能电池的电子供体和受体的分子工程对于开发具有改进的功率转换效率的新型材料至关重要。在这里,以非富勒烯受体(PTA-4F)和BTPTT-4F结合电子给体P2F-EHp为代表体系,从理论上研究了噻吩基融合到NFAs骨架中的作用。在广泛的量子化学计算的基础上,发现将噻吩基融合到主链中会提高最高占据分子轨道(HOMO)的能量,减小HOMO与最低未占据分子轨道之间的间隙,减小偶极矩,增强光强吸收和吸收带中产生的红移。P2F-EHp / BTPT-4F和P2F-EHp / BTPTT-4F配合物的数据表明,将噻吩基融合到电子受体的骨架中会改变供体/受体配合物的激发特性,增加电荷转移(CT)激发能量以及局部激发和CT杂交的低位激发态的数量,放大了CT速率,降低了激子解离速率,并显着抑制了电荷重组(CR)速率,从而提高了BTPTT-4F的性能。然而,BTPT-4F较差的性能主要归因于其更大的偶极矩和更快的CR率。增加电荷转移(CT)激发能以及局部激发和CT杂化低激发态的数量,放大CT速率,降低激子解离速率,并显着抑制电荷重组(CR)速率,从而提高性能BTPTT-4F。然而,BTPT-4F较差的性能主要归因于其更大的偶极矩和更快的CR率。增加电荷转移(CT)激发能以及局部激发和CT杂化低激发态的数量,放大CT速率,降低激子解离速率,并显着抑制电荷重组(CR)速率,从而提高性能BTPTT-4F。然而,BTPT-4F较差的性能主要归因于其更大的偶极矩和更快的CR率。
更新日期:2020-04-06
中文翻译:

噻吩在有机光伏异质结中融合到电子受体主链中:BTPT-4F和BTPTT-4F的比较研究
有机本体异质结太阳能电池的电子供体和受体的分子工程对于开发具有改进的功率转换效率的新型材料至关重要。在这里,以非富勒烯受体(PTA-4F)和BTPTT-4F结合电子给体P2F-EHp为代表体系,从理论上研究了噻吩基融合到NFAs骨架中的作用。在广泛的量子化学计算的基础上,发现将噻吩基融合到主链中会提高最高占据分子轨道(HOMO)的能量,减小HOMO与最低未占据分子轨道之间的间隙,减小偶极矩,增强光强吸收和吸收带中产生的红移。P2F-EHp / BTPT-4F和P2F-EHp / BTPTT-4F配合物的数据表明,将噻吩基融合到电子受体的骨架中会改变供体/受体配合物的激发特性,增加电荷转移(CT)激发能量以及局部激发和CT杂交的低位激发态的数量,放大了CT速率,降低了激子解离速率,并显着抑制了电荷重组(CR)速率,从而提高了BTPTT-4F的性能。然而,BTPT-4F较差的性能主要归因于其更大的偶极矩和更快的CR率。增加电荷转移(CT)激发能以及局部激发和CT杂化低激发态的数量,放大CT速率,降低激子解离速率,并显着抑制电荷重组(CR)速率,从而提高性能BTPTT-4F。然而,BTPT-4F较差的性能主要归因于其更大的偶极矩和更快的CR率。增加电荷转移(CT)激发能以及局部激发和CT杂化低激发态的数量,放大CT速率,降低激子解离速率,并显着抑制电荷重组(CR)速率,从而提高性能BTPTT-4F。然而,BTPT-4F较差的性能主要归因于其更大的偶极矩和更快的CR率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号