当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Non-covalent allosteric regulation of capsule catalysis
Chemical Science ( IF 7.6 ) Pub Date : 2020/03/02 , DOI: 10.1039/d0sc00341g Vicente Martí-Centelles 1, 2, 3, 4 , Rebecca L. Spicer 1, 2, 3, 4 , Paul J. Lusby 1, 2, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2020/03/02 , DOI: 10.1039/d0sc00341g Vicente Martí-Centelles 1, 2, 3, 4 , Rebecca L. Spicer 1, 2, 3, 4 , Paul J. Lusby 1, 2, 3, 4
Affiliation
|
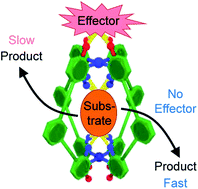
Allosteric regulation is an essential biological process that allows enzymes to modulate their active site properties by binding a control molecule at the protein exterior. Here we show the first example of capsule catalysis in which activity is changed by exotopic binding. This study utilizes a simple Pd2L4 capsule that can partition substrates and external effectors with high fidelity. We also present a detailed, quantitative understanding of how effector interactions alter both substrate and transition state binding. Unlike other allosteric host systems, perturbations are not a consequence of large mechanical changes, rather subtle electronic effects resulting from weak, non-covalent binding to the exterior surface. This investigation paves the way to more sophisticated allosteric systems.
中文翻译:

胶囊催化的非共价变构调节
变构调节是必不可少的生物学过程,它允许酶通过在蛋白质外部结合控制分子来调节其活性位点。在这里,我们显示了胶囊催化作用的第一个例子,其中活性通过外位结合而改变。这项研究利用了一个简单的Pd 2 L 4可以高保真度分隔基材和外部效应器的胶囊。我们还提出了关于效应子相互作用如何改变底物和过渡态结合的详细,定量的理解。与其他变构宿主系统不同,摄动不是大的机械变化的结果,而是微弱的,非共价结合到外表面的电子效应所导致的。这项研究为更复杂的变构系统铺平了道路。
更新日期:2020-03-26
中文翻译:

胶囊催化的非共价变构调节
变构调节是必不可少的生物学过程,它允许酶通过在蛋白质外部结合控制分子来调节其活性位点。在这里,我们显示了胶囊催化作用的第一个例子,其中活性通过外位结合而改变。这项研究利用了一个简单的Pd 2 L 4可以高保真度分隔基材和外部效应器的胶囊。我们还提出了关于效应子相互作用如何改变底物和过渡态结合的详细,定量的理解。与其他变构宿主系统不同,摄动不是大的机械变化的结果,而是微弱的,非共价结合到外表面的电子效应所导致的。这项研究为更复杂的变构系统铺平了道路。









































 京公网安备 11010802027423号
京公网安备 11010802027423号