Oncogene ( IF 6.9 ) Pub Date : 2020-03-02 , DOI: 10.1038/s41388-020-1235-2 Aaminah Khan 1, 2 , Emanuele Valli 1, 3 , Hayley Lam 1, 2 , David A Scott 4 , Jayne Murray 1 , Kimberley M Hanssen 1, 3 , Georgina Eden 1 , Laura D Gamble 1 , Rupinder Pandher 1 , Claudia L Flemming 1 , Sophie Allan 1 , Andrei L Osterman 4 , Michelle Haber 1 , Murray D Norris 1, 5 , Jamie I Fletcher 1, 3 , Denise M T Yu 1, 3
|
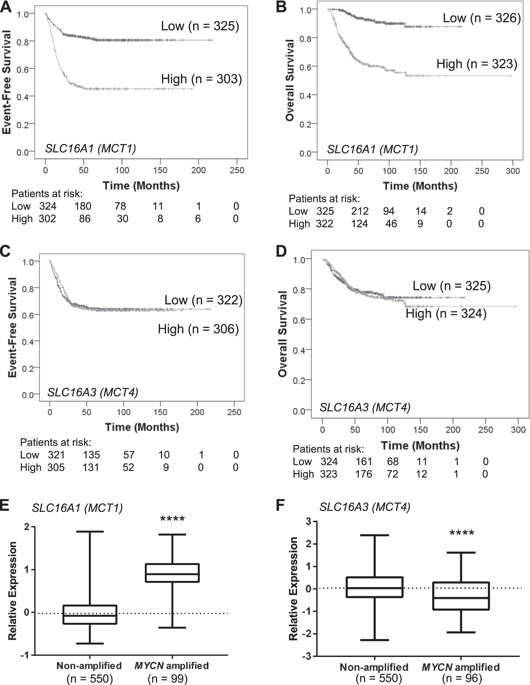
Amplification of the MYCN oncogene occurs in ~25% of primary neuroblastomas and is the single most powerful biological marker of poor prognosis in this disease. MYCN transcriptionally regulates a range of biological processes important for cancer, including cell metabolism. The MYCN-regulated metabolic gene SLC16A1, encoding the lactate transporter monocarboxylate transporter 1 (MCT1), is a potential therapeutic target. Treatment of neuroblastoma cells with the MCT1 inhibitor SR13800 increased intracellular lactate levels, disrupted the nicotinamide adenine dinucleotide (NADH/NAD+) ratio, and decreased intracellular glutathione levels. Metabolite tracing with 13C-glucose and 13C-glutamine following MCT1 inhibitor treatment revealed increased quantities of tricarboxylic acid (TCA) cycle intermediates and increased oxygen consumption rate. MCT1 inhibition was highly synergistic with vincristine and LDHA inhibition under cell culture conditions, but this combination was ineffective against neuroblastoma xenografts. Posttreatment xenograft tumors had increased synthesis of the MCT1 homolog MCT4/SLC16A, a known resistance factor to MCT1 inhibition. We found that MCT4 was negatively regulated by MYCN in luciferase reporter assays and its synthesis in neuroblastoma cells was increased under hypoxic conditions and following hypoxia-inducible factor (HIF1) induction, suggesting that MCT4 may contribute to resistance to MCT1 inhibitor treatment in hypoxic neuroblastoma tumors. Co-treatment of neuroblastoma cells with inhibitors of MCT1 and LDHA, the enzyme responsible for lactate production, resulted in a large increase in intracellular pyruvate and was highly synergistic in decreasing neuroblastoma cell viability. These results highlight the potential of targeting MCT1 in neuroblastoma in conjunction with strategies that involve disruption of pyruvate homeostasis and indicate possible resistance mechanisms.
中文翻译:

通过单羧酸转运蛋白 1 (MCT1) 抑制靶向高危神经母细胞瘤的代谢活动
MYCN癌基因的扩增发生在约 25% 的原发性神经母细胞瘤中,并且是该疾病预后不良的唯一最有力的生物学标志物。MYCN 转录调节一系列对癌症很重要的生物过程,包括细胞代谢。MYCN 调节的代谢基因SLC16A1编码乳酸转运蛋白单羧酸转运蛋白 1 (MCT1),是一个潜在的治疗靶点。用 MCT1 抑制剂 SR13800 治疗神经母细胞瘤细胞会增加细胞内乳酸水平,破坏烟酰胺腺嘌呤二核苷酸 (NADH/NAD +) 比率,并降低细胞内谷胱甘肽水平。在 MCT1 抑制剂处理后使用 13C-葡萄糖和 13C-谷氨酰胺进行代谢物追踪显示三羧酸 (TCA) 循环中间体数量增加和耗氧率增加。在细胞培养条件下,MCT1 抑制与长春新碱和 LDHA 抑制高度协同,但这种组合对神经母细胞瘤异种移植物无效。治疗后异种移植肿瘤增加了 MCT1 同源物 MCT4/ SLC16A的合成,这是一种已知的 MCT1 抑制抗性因子。我们发现 MCT4 受到MYCN的负调控在荧光素酶报告基因检测中,在缺氧条件下和缺氧诱导因子 (HIF1) 诱导后,其在神经母细胞瘤细胞中的合成增加,这表明 MCT4 可能有助于在缺氧神经母细胞瘤肿瘤中对 MCT1 抑制剂治疗产生耐药性。神经母细胞瘤细胞与 MCT1 抑制剂和负责乳酸产生的酶 LDHA 的共同处理导致细胞内丙酮酸的大量增加,并且在降低神经母细胞瘤细胞活力方面具有高度协同作用。这些结果突出了在神经母细胞瘤中靶向 MCT1 的潜力,以及涉及破坏丙酮酸稳态的策略并表明可能的耐药机制。











































 京公网安备 11010802027423号
京公网安备 11010802027423号