当前位置:
X-MOL 学术
›
Beilstein. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Two antibacterial and PPARα/γ-agonistic unsaturated keto fatty acids from a coral-associated actinomycete of the genus Micrococcus
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-02 , DOI: 10.3762/bjoc.16.29 Amit Raj Sharma , Enjuro Harunari , Naoya Oku , Nobuyasu Matsuura , Agus Trianto , Yasuhiro Igarashi
Beilstein Journal of Organic Chemistry ( IF 2.2 ) Pub Date : 2020-03-02 , DOI: 10.3762/bjoc.16.29 Amit Raj Sharma , Enjuro Harunari , Naoya Oku , Nobuyasu Matsuura , Agus Trianto , Yasuhiro Igarashi
|
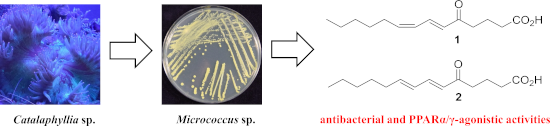
A pair of geometrically isomeric unsaturated keto fatty acids, (6E,8Z)- and (6E,8E)-5-oxo-6,8-tetradecadienoic acids (1 and 2), were isolated from the culture broth of an actinomycete of the genus Micrococcus, which was associated with a stony coral, Catalaphyllia sp. Their chemical structures were elucidated by spectroscopic analysis including NMR and MS, with special assistance of spin system simulation studies for the assignment of an E geometry at C8 in 2. As metabolites of microbes, compounds 1 and 2 are unprecedented in terms of bearing a 2,4-dienone system. Both 1 and 2 showed antibacterial activity against the plant pathogen Rhizobium radiobacter and the fish pathogen Tenacibaculum maritimum, with a contrasting preference that 1 is more effective to the former strain while 2 is so to the latter. In addition, compounds 1 and 2 displayed agonistic activity against peroxisome proliferator-activated receptors (PPARs) with an isoform specificity towards PPARα and PPARγ.
中文翻译:

来自微球菌属的珊瑚相关放线菌的两种抗菌和PPARα/γ激动性不饱和酮脂肪酸
从的培养液中分离了一对几何异构的不饱和酮脂肪酸(6 E,8 Z)和(6 E,8 E)-5-氧代-6,8-十四碳二烯酸(1和2)。Micrococcus属的放线菌,与石质珊瑚Catalaphyllia sp。相关。通过包括NMR和MS在内的光谱分析阐明了它们的化学结构,并特别借助自旋系统模拟研究对C8 in 2中的E几何进行了赋值。作为微生物的代谢产物,化合物1和2在承载2,4-二烯酮体系方面是前所未有的。既1和2显示出对植物病原体的抗菌活性根瘤菌放射形和鱼病原体黏着maritimum,具有形成对比的首选项1更有效的是前者应变而2是这样后者。此外,化合物1和2对过氧化物酶体增殖物激活的受体(PPAR)表现出激动活性,并且对PPARα和PPARγ具有同种型特异性。
更新日期:2020-03-02
中文翻译:

来自微球菌属的珊瑚相关放线菌的两种抗菌和PPARα/γ激动性不饱和酮脂肪酸
从的培养液中分离了一对几何异构的不饱和酮脂肪酸(6 E,8 Z)和(6 E,8 E)-5-氧代-6,8-十四碳二烯酸(1和2)。Micrococcus属的放线菌,与石质珊瑚Catalaphyllia sp。相关。通过包括NMR和MS在内的光谱分析阐明了它们的化学结构,并特别借助自旋系统模拟研究对C8 in 2中的E几何进行了赋值。作为微生物的代谢产物,化合物1和2在承载2,4-二烯酮体系方面是前所未有的。既1和2显示出对植物病原体的抗菌活性根瘤菌放射形和鱼病原体黏着maritimum,具有形成对比的首选项1更有效的是前者应变而2是这样后者。此外,化合物1和2对过氧化物酶体增殖物激活的受体(PPAR)表现出激动活性,并且对PPARα和PPARγ具有同种型特异性。







































 京公网安备 11010802027423号
京公网安备 11010802027423号