当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hyperbranched lipoid-based lipid nanoparticles for bidirectional regulation of collagen accumulation in liver fibrosis.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.jconrel.2020.02.049 Jian-Bin Qiao 1 , Qian-Qian Fan 2 , Cheng-Lu Zhang 2 , Jaiwoo Lee 3 , Junho Byun 3 , Lei Xing 4 , Xiang-Dong Gao 5 , Yu-Kyoung Oh 3 , Hu-Lin Jiang 4
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.jconrel.2020.02.049 Jian-Bin Qiao 1 , Qian-Qian Fan 2 , Cheng-Lu Zhang 2 , Jaiwoo Lee 3 , Junho Byun 3 , Lei Xing 4 , Xiang-Dong Gao 5 , Yu-Kyoung Oh 3 , Hu-Lin Jiang 4
Affiliation
|
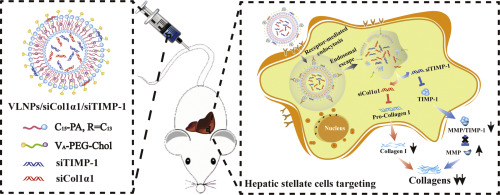
Liver fibrosis leads to over one million deaths annually worldwide. Hepatic stellate cells (HSCs) have been identified as the main executors of liver fibrosis. Unfortunately, no drug has yet been approved for clinical use against liver fibrosis, largely because the tested drugs have been unable to access HSCs and efficiently remove the collagen accumulation involved in fibrogenesis. Here, we designed an efficient HSC-targeting lipid delivery system that carried dual siRNAs intended to both inhibit collagen synthesis and promote collagen degradation, with the goal of realizing enhanced anti-liver fibrosis by bidirectional regulation of collagen accumulation. The delivery system was constructed by using amphiphilic cationic hyperbranched lipoids (C15-PA) for siRNA complexation and helper lipoids (cholesterol-polyethylene glycol-vitamin A, Chol-PEG-VA) for HSCs targeting. The generated vitamin A-decorated and hyperbranched lipoid-based lipid nanoparticles (VLNPs) showed excellent gene-binding ability and transfection efficiency, and enhanced the delivery of siRNAs to HSCs. Fibrotic mice treated with dual siRNA-loaded VLNPs showed a great reduction in the collagen accumulation seen in this model; the enhanced effect of bidirectional regulation reduced the collagen accumulation level in treated mice to almost that seen in normal mice. There was no notable sign of toxicity or tissue inflammation in mice exposed to repeated intravenous administration of the dual siRNA-loaded VLNPs. In conclusion, our results indicate that biocompatible VLNPs designed to exploit precise targeting and an effective bidirectional regulation strategy hold promise for treating liver fibrosis.
中文翻译:

超支化脂质基脂质纳米颗粒,可双向调节肝纤维化中胶原蛋白的积累。
肝纤维化导致全世界每年超过一百万的死亡。肝星状细胞(HSC)已被确定为肝纤维化的主要执行者。不幸的是,尚未有任何药物被批准用于临床抗肝纤维化,这主要是因为被测试的药物无法进入HSCs并不能有效地去除涉及纤维化的胶原蛋白积累。在这里,我们设计了一种高效的靶向HSC的脂质递送系统,该系统携带旨在抑制胶原蛋白合成和促进胶原蛋白降解的双重siRNA,目的是通过胶原蛋白积累的双向调节来实现增强的抗肝脏纤维化。使用两亲性阳离子超支化脂质(C15-PA)和siRNA辅助脂质(胆固醇-聚乙二醇-维生素A,用于HSC靶向的Chol-PEG-VA)。生成的维生素A装饰和超支化脂质基脂质纳米颗粒(VLNPs)具有出色的基因结合能力和转染效率,并增强了siRNA向HSC的传递。用双siRNA加载的VLNP处理的纤维化小鼠在该模型中显示出胶原蛋白积聚的极大减少。双向调节作用的增强将治疗小鼠的胶原蛋白积累水平降低到几乎与正常小鼠相同。在反复静脉内施用双重siRNA加载的VLNP的小鼠中,没有明显的毒性或组织炎症迹象。总之,我们的结果表明,旨在开发精确靶向和有效双向调节策略的生物相容性VLNP具有治疗肝纤维化的希望。
更新日期:2020-03-02
中文翻译:

超支化脂质基脂质纳米颗粒,可双向调节肝纤维化中胶原蛋白的积累。
肝纤维化导致全世界每年超过一百万的死亡。肝星状细胞(HSC)已被确定为肝纤维化的主要执行者。不幸的是,尚未有任何药物被批准用于临床抗肝纤维化,这主要是因为被测试的药物无法进入HSCs并不能有效地去除涉及纤维化的胶原蛋白积累。在这里,我们设计了一种高效的靶向HSC的脂质递送系统,该系统携带旨在抑制胶原蛋白合成和促进胶原蛋白降解的双重siRNA,目的是通过胶原蛋白积累的双向调节来实现增强的抗肝脏纤维化。使用两亲性阳离子超支化脂质(C15-PA)和siRNA辅助脂质(胆固醇-聚乙二醇-维生素A,用于HSC靶向的Chol-PEG-VA)。生成的维生素A装饰和超支化脂质基脂质纳米颗粒(VLNPs)具有出色的基因结合能力和转染效率,并增强了siRNA向HSC的传递。用双siRNA加载的VLNP处理的纤维化小鼠在该模型中显示出胶原蛋白积聚的极大减少。双向调节作用的增强将治疗小鼠的胶原蛋白积累水平降低到几乎与正常小鼠相同。在反复静脉内施用双重siRNA加载的VLNP的小鼠中,没有明显的毒性或组织炎症迹象。总之,我们的结果表明,旨在开发精确靶向和有效双向调节策略的生物相容性VLNP具有治疗肝纤维化的希望。











































 京公网安备 11010802027423号
京公网安备 11010802027423号