当前位置:
X-MOL 学术
›
J. Control. Release
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Programmable antibiotic delivery to combat methicillin-resistant Staphylococcus aureus through precision therapy.
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.jconrel.2020.02.048 Shaoqi Qu 1 , Ying Liu 1 , Qiao Hu 1 , Yiming Han 2 , Zhihui Hao 1 , Jianzhong Shen 3 , Kui Zhu 1
Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.jconrel.2020.02.048 Shaoqi Qu 1 , Ying Liu 1 , Qiao Hu 1 , Yiming Han 2 , Zhihui Hao 1 , Jianzhong Shen 3 , Kui Zhu 1
Affiliation
|
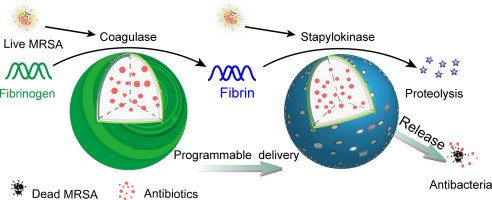
The rapid dissemination of life-threatening multidrug-resistant bacterial pathogens calls for the development of new antibacterial agents and alternative strategies. The virulence factor secreted by bacteria plays a crucial role in the sophisticated processes during infections. Inspired by the unique capacity of many bacteria inducing clotting of plasma to initiate colonization, we propose a programmable antibiotic delivery system for precision therapy using methicillin-resistant S. aureus (MRSA) as a model. Coagulase utilized by MRSA to directly cleave fibrinogen into fibrin, is an ideal target not only for tracking bacterial status but for triggering the collapse of fibrinogen functionalized porous microspheres. Subsequently, staphylokinase, another virulence factor of MRSA, catalyzed hydrolysis of fibrin to further release the encapsulated antibiotics from microspheres. Our sequential triggered-release system exhibits high selectivity to distinguish live or dead MRSA from other pathogenic bacteria. Furthermore, such programmable microspheres clear 99% MRSA in 4 h, and show increased efficiency in a wound healing model in rats. Our study provides a programmable drug delivery system to precisely target bacterial pathogens using their intrinsic enzymatic cascades. This programmable platform with reduced selective stress of antibiotics on microbiota sheds light on the potential therapy for future clinical applications.
中文翻译:

通过精确治疗可通过可编程的抗生素递送抗击耐甲氧西林的金黄色葡萄球菌。
快速传播威胁生命的耐多药细菌病原体,要求开发新的抗菌剂和替代策略。细菌分泌的毒力因子在感染过程中的复杂过程中起着至关重要的作用。受许多细菌诱导血浆凝集以启动定植的独特能力的启发,我们提出了一种可编程的抗生素输送系统,用于耐甲氧西林的金黄色葡萄球菌(MRSA)作为模型的精密治疗。MRSA利用凝固酶直接将血纤蛋白原裂解为血纤蛋白,不仅是追踪细菌状态,而且是触发血纤蛋白原功能化的多孔微球体崩溃的理想靶标。随后,葡萄球菌激酶(MRSA的另一种毒力因子)催化的纤维蛋白水解,进一步从微球中释放出包封的抗生素。我们的顺序触发释放系统具有很高的选择性,可以将活的或死亡的MRSA与其他病原细菌区分开。此外,此类可编程微球可在4小时内清除99%MRSA,并在大鼠伤口愈合模型中显示出更高的效率。我们的研究提供了一种可编程的药物输送系统,可使用其固有的酶促级联反应精确靶向细菌病原体。这个可编程平台降低了微生物对抗生素的选择性压力,为将来的临床应用提供了潜在的治疗方法。这样的可编程微球可在4小时内清除99%MRSA,并在大鼠伤口愈合模型中显示出更高的效率。我们的研究提供了一种可编程的药物输送系统,可使用其固有的酶促级联反应精确靶向细菌病原体。这个可编程平台减少了微生物对抗生素的选择性压力,为将来的临床应用提供了潜在的疗法。这样的可编程微球可在4小时内清除99%MRSA,并在大鼠伤口愈合模型中显示出更高的效率。我们的研究提供了一种可编程的药物输送系统,可使用其固有的酶促级联反应精确靶向细菌病原体。这个可编程平台降低了微生物对抗生素的选择性压力,为将来的临床应用提供了潜在的治疗方法。
更新日期:2020-03-02
中文翻译:

通过精确治疗可通过可编程的抗生素递送抗击耐甲氧西林的金黄色葡萄球菌。
快速传播威胁生命的耐多药细菌病原体,要求开发新的抗菌剂和替代策略。细菌分泌的毒力因子在感染过程中的复杂过程中起着至关重要的作用。受许多细菌诱导血浆凝集以启动定植的独特能力的启发,我们提出了一种可编程的抗生素输送系统,用于耐甲氧西林的金黄色葡萄球菌(MRSA)作为模型的精密治疗。MRSA利用凝固酶直接将血纤蛋白原裂解为血纤蛋白,不仅是追踪细菌状态,而且是触发血纤蛋白原功能化的多孔微球体崩溃的理想靶标。随后,葡萄球菌激酶(MRSA的另一种毒力因子)催化的纤维蛋白水解,进一步从微球中释放出包封的抗生素。我们的顺序触发释放系统具有很高的选择性,可以将活的或死亡的MRSA与其他病原细菌区分开。此外,此类可编程微球可在4小时内清除99%MRSA,并在大鼠伤口愈合模型中显示出更高的效率。我们的研究提供了一种可编程的药物输送系统,可使用其固有的酶促级联反应精确靶向细菌病原体。这个可编程平台降低了微生物对抗生素的选择性压力,为将来的临床应用提供了潜在的治疗方法。这样的可编程微球可在4小时内清除99%MRSA,并在大鼠伤口愈合模型中显示出更高的效率。我们的研究提供了一种可编程的药物输送系统,可使用其固有的酶促级联反应精确靶向细菌病原体。这个可编程平台减少了微生物对抗生素的选择性压力,为将来的临床应用提供了潜在的疗法。这样的可编程微球可在4小时内清除99%MRSA,并在大鼠伤口愈合模型中显示出更高的效率。我们的研究提供了一种可编程的药物输送系统,可使用其固有的酶促级联反应精确靶向细菌病原体。这个可编程平台降低了微生物对抗生素的选择性压力,为将来的临床应用提供了潜在的治疗方法。











































 京公网安备 11010802027423号
京公网安备 11010802027423号