Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
FBXL5 Regulates IRP2 Stability in Iron Homeostasis via an Oxygen-Responsive [2Fe2S] Cluster.
Molecular Cell ( IF 14.5 ) Pub Date : 2020-02-25 , DOI: 10.1016/j.molcel.2020.02.011 Hui Wang 1 , Hui Shi 1 , Malini Rajan 2 , Elizabeth R Canarie 3 , Seoyeon Hong 3 , Daniele Simoneschi 4 , Michele Pagano 5 , Matthew F Bush 3 , Stefan Stoll 3 , Elizabeth A Leibold 2 , Ning Zheng 1
Molecular Cell ( IF 14.5 ) Pub Date : 2020-02-25 , DOI: 10.1016/j.molcel.2020.02.011 Hui Wang 1 , Hui Shi 1 , Malini Rajan 2 , Elizabeth R Canarie 3 , Seoyeon Hong 3 , Daniele Simoneschi 4 , Michele Pagano 5 , Matthew F Bush 3 , Stefan Stoll 3 , Elizabeth A Leibold 2 , Ning Zheng 1
Affiliation
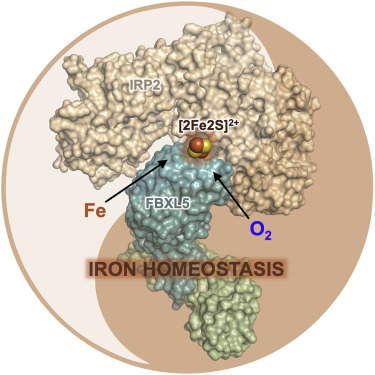
|
Cellular iron homeostasis is dominated by FBXL5-mediated degradation of iron regulatory protein 2 (IRP2), which is dependent on both iron and oxygen. However, how the physical interaction between FBXL5 and IRP2 is regulated remains elusive. Here, we show that the C-terminal substrate-binding domain of FBXL5 harbors a [2Fe2S] cluster in the oxidized state. A cryoelectron microscopy (cryo-EM) structure of the IRP2-FBXL5-SKP1 complex reveals that the cluster organizes the FBXL5 C-terminal loop responsible for recruiting IRP2. Interestingly, IRP2 binding to FBXL5 hinges on the oxidized state of the [2Fe2S] cluster maintained by ambient oxygen, which could explain hypoxia-induced IRP2 stabilization. Steric incompatibility also allows FBXL5 to physically dislodge IRP2 from iron-responsive element RNA to facilitate its turnover. Taken together, our studies have identified an iron-sulfur cluster within FBXL5, which promotes IRP2 polyubiquitination and degradation in response to both iron and oxygen concentrations.
中文翻译:

FBXL5 通过氧响应性 [2Fe2S] 簇调节 IRP2 在铁稳态中的稳定性。
细胞铁稳态主要由 FBXL5 介导的铁调节蛋白 2 (IRP2) 降解决定,这依赖于铁和氧。然而,如何调节 FBXL5 和 IRP2 之间的物理相互作用仍然难以捉摸。在这里,我们展示了 FBXL5 的 C 末端底物结合域含有一个处于氧化状态的 [2Fe2S] 簇。IRP2-FBXL5-SKP1 复合物的低温电子显微镜 (cryo-EM) 结构显示该簇组织了负责招募 IRP2 的 FBXL5 C 端环。有趣的是,IRP2 与 FBXL5 的结合取决于环境氧维持的 [2Fe2S] 簇的氧化状态,这可以解释缺氧诱导的 IRP2 稳定化。空间不相容性还允许 FBXL5 物理地将 IRP2 从铁响应元件 RNA 中移出,以促进其周转。综合起来,
更新日期:2020-03-02
中文翻译:

FBXL5 通过氧响应性 [2Fe2S] 簇调节 IRP2 在铁稳态中的稳定性。
细胞铁稳态主要由 FBXL5 介导的铁调节蛋白 2 (IRP2) 降解决定,这依赖于铁和氧。然而,如何调节 FBXL5 和 IRP2 之间的物理相互作用仍然难以捉摸。在这里,我们展示了 FBXL5 的 C 末端底物结合域含有一个处于氧化状态的 [2Fe2S] 簇。IRP2-FBXL5-SKP1 复合物的低温电子显微镜 (cryo-EM) 结构显示该簇组织了负责招募 IRP2 的 FBXL5 C 端环。有趣的是,IRP2 与 FBXL5 的结合取决于环境氧维持的 [2Fe2S] 簇的氧化状态,这可以解释缺氧诱导的 IRP2 稳定化。空间不相容性还允许 FBXL5 物理地将 IRP2 从铁响应元件 RNA 中移出,以促进其周转。综合起来,











































 京公网安备 11010802027423号
京公网安备 11010802027423号