Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-03-02 , DOI: 10.1016/j.bbamem.2020.183252 Sébastien Deshayes 1 , Karidia Konate 1 , Marion Dussot 1 , Bérengère Chavey 2 , Anaïs Vaissière 1 , Thi Nhu Ngoc Van 3 , Gudrun Aldrian 3 , Kärt Padari 4 , Margus Pooga 5 , Eric Vivès 1 , Prisca Boisguérin 1
|
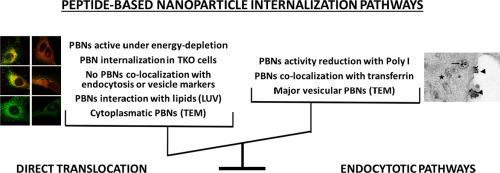
Gene silencing mediated by double-stranded small interfering RNA (siRNA) has been widely investigated as a potential therapeutic approach for a variety of diseases and, indeed, the first therapeutic siRNA was approved by the FDA in 2018. As an alternative to the traditional delivery systems for nucleic acids, peptide-based nanoparticles (PBNs) have been applied successfully for siRNA delivery. Recently, we have developed amphipathic cell-penetrating peptides (CPPs), called WRAP allowing a rapid and efficient siRNA delivery into several cell lines at low doses (20 to 50 nM).
In this study, using a highly specific gene silencing system, we aimed to elucidate the cellular uptake mechanism of WRAP:siRNA nanoparticles by combining biophysical, biological, confocal and electron microscopy approaches. We demonstrated that WRAP:siRNA complexes remain fully active in the presence of chemical inhibitors of different endosomal pathways suggesting a direct cell membrane translocation mechanism. Leakage studies on lipid vesicles indicated membrane destabilization properties of the nanoparticles and this was supported by the measurement of WRAP:siRNA internalization in dynamin triple-KO cells. However, we also observed some evidences for an endocytosis-dependent cellular internalization. Indeed, nanoparticles co-localized with transferrin, siRNA silencing was inhibited by the scavenger receptor A inhibitor Poly I and nanoparticles encapsulated in vesicles were observed by electron microscopy in U87 cells.
In conclusion, we demonstrate here that the efficiency of WRAP:siRNA nanoparticles is mainly based on the use of multiple internalization mechanisms including direct translocation as well as endocytosis-dependent pathways.
中文翻译:

破译WRAP:siRNA纳米粒子的内部化机制。
由双链小干扰RNA(siRNA)介导的基因沉默已被广泛研究为多种疾病的潜在治疗方法,实际上,首个治疗性siRNA已于2018年获得FDA批准。作为传统药物的替代方法用于核酸的系统中,基于肽的纳米颗粒(PBN)已成功应用于siRNA递送。最近,我们已经开发出称为WRAP的两亲性细胞穿透肽(CPPs),可将低剂量(20至50 nM)的siRNA快速有效地递送到多种细胞系中。
在这项研究中,我们使用高度特异性的基因沉默系统,旨在通过结合生物物理,生物学,共聚焦和电子显微镜方法阐明WRAP:siRNA纳米颗粒的细胞摄取机制。我们证明了WRAP:siRNA复合物在存在不同内体途径化学抑制剂的情况下仍保持完全活性,表明存在直接的细胞膜易位机制。对脂质囊泡的渗漏研究表明纳米颗粒具有膜失稳特性,这通过在动态三联KO细胞中WRAP:siRNA内在化的测量得到支持。但是,我们也观察到了一些内吞作用依赖性细胞内在化的证据。实际上,纳米颗粒与运铁蛋白共定位,
总之,我们在这里证明WRAP:siRNA纳米颗粒的效率主要基于多种内在化机制的使用,包括直接易位以及胞吞作用依赖性途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号