当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Isotope Effects: Interpretation and Prediction Using Degrees of Rate Control
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-19 , DOI: 10.1021/acscatal.9b05637 Zhongtian Mao 1 , Charles T. Campbell 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-19 , DOI: 10.1021/acscatal.9b05637 Zhongtian Mao 1 , Charles T. Campbell 1
Affiliation
|
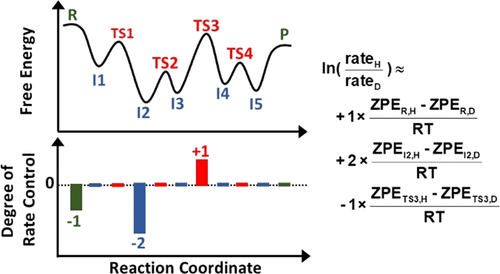
Kinetic isotope effects (KIEs) have been used for decades in catalysis research as a tool for clarifying reaction mechanisms. Significant primary kinetic isotope effects have usually been interpreted as being a result of isotope substitution at a site of bond breaking (or forming) in the rate-determining step in the reaction mechanism. However, quantitative analysis of the magnitude of the KIE in complex multistep reaction mechanisms is seldom reported. We prove here that the logarithm of the rate ratio for two isotopes is the weighted average over all species in the mechanism of their standard-state free-energy difference between the two isotopes, divided by RT. The weighting factor is the degree of rate control (DRC) for that species (e.g., transition state, intermediate, reactant) when the rate is measured separately for each isotope. It is instead the degree of selectivity ratio control (DSRC) when the KIE is measured as the product selectivity in a parallel competition between two isotopes within the same reactant molecule. Since only a few species have nonzero DRCs (or DSRCs) for most reactions, only these few contribute to this weighted average and the KIE. We show that this provides a simple way to interpret and quantitatively predict kinetic isotope effects that is powerful in the insights it provides, allowing one to evaluate directly which species contribute most to the KIE. By applying it to H/D KIEs in several example mechanisms, we further show that the traditional way of interpreting KIEs that focuses only on the rate-determining step can easily lead to misunderstanding of KIE and the reaction mechanism. This highlights the importance to consider the effect of isotope substitution on the energies of all species with large DRCs (i.e., those whose energies are kinetically relevant). This method also offers opportunities for quantitative validation of mechanism-based microkinetic models.
中文翻译:

动力学同位素效应:使用速率控制度的解释和预测
动力学同位素效应(KIEs)已在催化研究中用作阐明反应机理的工具已有数十年的历史了。显着的主要动力学同位素效应通常被解释为在反应机理的速率确定步骤中,在键断裂(或形成)的位点被同位素取代的结果。然而,很少有关于复杂多步反应机理中KIE量的定量分析的报道。我们在这里证明两种同位素比率比率的对数是两种同位素的标准态自由能差机制中所有物种的加权平均值,除以RT。权重因子是当对每种同位素分别测量速率时该物种(例如过渡态,中间体,反应物)的速率控制(DRC)程度。取而代之的是,当将KIE作为同一反应物分子内两个同位素之间平行竞争中的产物选择性进行测量时,它是选择性比率控制的程度(DSRC)。由于对于大多数反应而言,只有少数物种具有非零的DRC(或DSRC),因此只有极少数物种对该加权平均值和KIE有所贡献。我们表明,这提供了一种解释和定量预测动力学同位素效应的简单方法,该方法在其提供的见解中具有强大的作用,使人们可以直接评估哪些物种对KIE的贡献最大。通过几种示例机制将其应用于H / D KIE,我们进一步表明,仅关注速率确定步骤的传统解释KIE的方式很容易导致对KIE和反应机制的误解。这突出了考虑同位素取代对所有具有大DRC的物种(即那些能量与动力学相关的物种)的能量的影响的重要性。这种方法也为定量验证基于机理的微动力学模型提供了机会。
更新日期:2020-03-20
中文翻译:

动力学同位素效应:使用速率控制度的解释和预测
动力学同位素效应(KIEs)已在催化研究中用作阐明反应机理的工具已有数十年的历史了。显着的主要动力学同位素效应通常被解释为在反应机理的速率确定步骤中,在键断裂(或形成)的位点被同位素取代的结果。然而,很少有关于复杂多步反应机理中KIE量的定量分析的报道。我们在这里证明两种同位素比率比率的对数是两种同位素的标准态自由能差机制中所有物种的加权平均值,除以RT。权重因子是当对每种同位素分别测量速率时该物种(例如过渡态,中间体,反应物)的速率控制(DRC)程度。取而代之的是,当将KIE作为同一反应物分子内两个同位素之间平行竞争中的产物选择性进行测量时,它是选择性比率控制的程度(DSRC)。由于对于大多数反应而言,只有少数物种具有非零的DRC(或DSRC),因此只有极少数物种对该加权平均值和KIE有所贡献。我们表明,这提供了一种解释和定量预测动力学同位素效应的简单方法,该方法在其提供的见解中具有强大的作用,使人们可以直接评估哪些物种对KIE的贡献最大。通过几种示例机制将其应用于H / D KIE,我们进一步表明,仅关注速率确定步骤的传统解释KIE的方式很容易导致对KIE和反应机制的误解。这突出了考虑同位素取代对所有具有大DRC的物种(即那些能量与动力学相关的物种)的能量的影响的重要性。这种方法也为定量验证基于机理的微动力学模型提供了机会。











































 京公网安备 11010802027423号
京公网安备 11010802027423号