当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Combination of Biocompatible Room Temperature Ionic Liquid and Palladium Catalyst for Base‐ and Ligand‐Free Suzuki Coupling Reactions
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-03-12 , DOI: 10.1002/ajoc.202000059 Seong‐Ryu Joo 1 , Gyu‐Tae Kwon 1 , Seung‐Hoi Kim 1
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-03-12 , DOI: 10.1002/ajoc.202000059 Seong‐Ryu Joo 1 , Gyu‐Tae Kwon 1 , Seung‐Hoi Kim 1
Affiliation
|
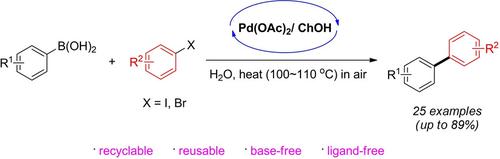
A system with mild and versatile reaction conditions for a carbon–carbon bond‐forming reaction using arylboronic acids and aryl halides was developed. A readily available and biodegradable room‐temperature ionic liquid, choline hydroxide (ChOH), was combined with a ligand‐free Pd(OAc)2‐catalyst, providing the corresponding symmetrical and/or unsymmetrical biaryl products in satisfactory yields under aerobic conditions. No external base or ligand was required for completion of the cross‐coupling reactions. More significantly, the reaction medium showed good recyclability, which is an important characteristic from the viewpoint of sustainable chemistry.
中文翻译:

生物相容性室温离子液体和钯催化剂的组合,用于无碱和无配体的铃木偶联反应
开发了具有温和通用反应条件的系统,该系统可使用芳基硼酸和芳基卤化物进行碳-碳键形成反应。将易于获得且可生物降解的室温离子液体氢氧化胆碱(ChOH)与无配体的Pd(OAc)2催化剂混合,在好氧条件下以令人满意的收率提供相应的对称和/或不对称联芳基产品。完成交叉偶联反应不需要外部碱或配体。更重要的是,反应介质显示出良好的可循环性,这从可持续化学的观点来看是重要的特征。
更新日期:2020-04-21
中文翻译:

生物相容性室温离子液体和钯催化剂的组合,用于无碱和无配体的铃木偶联反应
开发了具有温和通用反应条件的系统,该系统可使用芳基硼酸和芳基卤化物进行碳-碳键形成反应。将易于获得且可生物降解的室温离子液体氢氧化胆碱(ChOH)与无配体的Pd(OAc)2催化剂混合,在好氧条件下以令人满意的收率提供相应的对称和/或不对称联芳基产品。完成交叉偶联反应不需要外部碱或配体。更重要的是,反应介质显示出良好的可循环性,这从可持续化学的观点来看是重要的特征。









































 京公网安备 11010802027423号
京公网安备 11010802027423号