当前位置:
X-MOL 学术
›
Eur. J. Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Human T cells employ conserved AU-rich elements to fine-tune IFN-γ production.
European Journal of Immunology ( IF 4.5 ) Pub Date : 2020-02-29 , DOI: 10.1002/eji.201948458 Julian J Freen-van Heeren 1, 2 , Branka Popović 1, 2 , Aurélie Guislain 1, 2 , Monika C Wolkers 1, 2
European Journal of Immunology ( IF 4.5 ) Pub Date : 2020-02-29 , DOI: 10.1002/eji.201948458 Julian J Freen-van Heeren 1, 2 , Branka Popović 1, 2 , Aurélie Guislain 1, 2 , Monika C Wolkers 1, 2
Affiliation
|
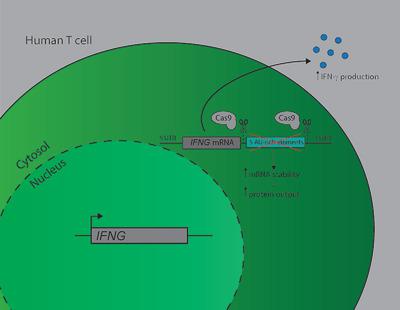
Long‐lasting CD8+ T cell responses are critical in combatting infections and tumors. The pro‐inflammatory cytokine IFN‐γ is a key effector molecule herein. We recently showed that in murine T cells the production of IFN‐γ is tightly regulated through adenylate uridylate–rich elements (AREs) that are located in the 3′ untranslated region (UTR) of the Ifng mRNA molecule. Loss of AREs resulted in prolonged cytokine production in activated T cells and boosted anti‐tumoral T cell responses. Here, we investigated whether these findings can be translated to primary human T cells. Utilizing CRISPR‐Cas9 technology, we deleted the ARE region from the IFNG 3′ UTR in peripheral blood‐derived human T cells. Loss of AREs stabilized the IFNG mRNA in T cells and supported a higher proportion of IFN‐γ protein‐producing T cells. Importantly, combining MART‐1 T cell receptor engineering with ARE‐Del gene editing showed that this was also true for antigen‐specific activation of T cells. MART‐1‐specific ARE‐Del T cells showed higher percentages of IFN‐γ producing T cells in response to MART‐1 expressing tumor cells. Combined, our study reveals that ARE‐mediated posttranscriptional regulation is conserved between murine and human T cells. Furthermore, generating antigen‐specific ARE‐Del T cells is feasible, a feature that could potentially be used for therapeutical purposes.
中文翻译:

人T细胞利用保守的富含AU的元素来微调IFN-γ的产生。
持久的CD8 + T细胞反应对于抵抗感染和肿瘤至关重要。促炎细胞因子IFN-γ是本文的关键效应分子。我们最近发现,在鼠T细胞中,IFN-γ的产生通过位于Ifng mRNA分子3'非翻译区(UTR)中的富含腺苷酸尿苷酸的元件(ARE)受到严格调节。ARE的缺失导致活化T细胞中细胞因子的产生延长,并增强了抗肿瘤T细胞反应。在这里,我们调查了这些发现是否可以翻译成原代人T细胞。利用CRISPR-Cas9技术,我们从外周血源性人类T细胞中的IFNG 3'UTR中删除了ARE区。ARE的丢失稳定了IFNGT细胞中的mRNA和支持更高比例的产生IFN-γ蛋白的T细胞。重要的是,将MART-1 T细胞受体工程技术与ARE-Del基因编辑相结合表明,对于T细胞的抗原特异性激活也是如此。MART-1特异的ARE-Del T细胞对表达MART-1的肿瘤细胞表现出更高的产生IFN-γ的T细胞百分比。综合来看,我们的研究表明,鼠和人T细胞之间ARE介导的转录后调控是保守的。此外,生成抗原特异性ARE-Del T细胞是可行的,该功能可能会用于治疗目的。
更新日期:2020-02-29
中文翻译:

人T细胞利用保守的富含AU的元素来微调IFN-γ的产生。
持久的CD8 + T细胞反应对于抵抗感染和肿瘤至关重要。促炎细胞因子IFN-γ是本文的关键效应分子。我们最近发现,在鼠T细胞中,IFN-γ的产生通过位于Ifng mRNA分子3'非翻译区(UTR)中的富含腺苷酸尿苷酸的元件(ARE)受到严格调节。ARE的缺失导致活化T细胞中细胞因子的产生延长,并增强了抗肿瘤T细胞反应。在这里,我们调查了这些发现是否可以翻译成原代人T细胞。利用CRISPR-Cas9技术,我们从外周血源性人类T细胞中的IFNG 3'UTR中删除了ARE区。ARE的丢失稳定了IFNGT细胞中的mRNA和支持更高比例的产生IFN-γ蛋白的T细胞。重要的是,将MART-1 T细胞受体工程技术与ARE-Del基因编辑相结合表明,对于T细胞的抗原特异性激活也是如此。MART-1特异的ARE-Del T细胞对表达MART-1的肿瘤细胞表现出更高的产生IFN-γ的T细胞百分比。综合来看,我们的研究表明,鼠和人T细胞之间ARE介导的转录后调控是保守的。此外,生成抗原特异性ARE-Del T细胞是可行的,该功能可能会用于治疗目的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号