当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Mannich‐Type Reaction of Malononitrile with N‐Boc α‐Ketiminoesters Using Chiral Organic Base Catalyst with Halogen Bond Donor Functionality
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-13 , DOI: 10.1002/adsc.202000092 Satoru Kuwano 1 , Yuki Nishida 1 , Takumi Suzuki 1 , Takayoshi Arai 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2020-03-13 , DOI: 10.1002/adsc.202000092 Satoru Kuwano 1 , Yuki Nishida 1 , Takumi Suzuki 1 , Takayoshi Arai 1
Affiliation
|
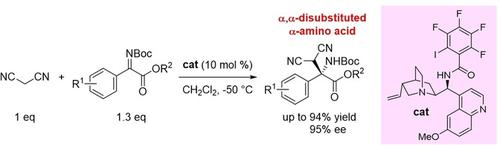
Chiral organic base catalyst with halogen bond donor functionality catalyzed asymmetric Mannich‐type reaction of malononitrile with α‐ketiminoesters to produce α,α‐disubstituted α‐amino acid derivatives in good yields with high enantioselectivities. The malononitrile‐derived amino acid was smoothly transformed to chiral aminomalonate without loss of ee.
中文翻译:

使用具有卤素键供体功能的手性有机碱催化剂催化丙二腈与N-Bocα-酮亚胺酸酯的不对称曼尼希型反应
具有卤素键供体功能的手性有机碱催化剂催化丙二腈与α-酮亚氨基酸酯的不对称曼尼希型反应,以高收率和高对映选择性生产α,α-二取代α-氨基酸衍生物。丙二腈衍生的氨基酸被平滑地转化为手性氨基丙二酸酯,而不会损失ee。
更新日期:2020-04-21
中文翻译:

使用具有卤素键供体功能的手性有机碱催化剂催化丙二腈与N-Bocα-酮亚胺酸酯的不对称曼尼希型反应
具有卤素键供体功能的手性有机碱催化剂催化丙二腈与α-酮亚氨基酸酯的不对称曼尼希型反应,以高收率和高对映选择性生产α,α-二取代α-氨基酸衍生物。丙二腈衍生的氨基酸被平滑地转化为手性氨基丙二酸酯,而不会损失ee。











































 京公网安备 11010802027423号
京公网安备 11010802027423号