Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An adaptable implementation package targeting evidence-based indicators in primary care: A pragmatic cluster-randomised evaluation.
PLOS Medicine ( IF 15.8 ) Pub Date : 2020-02-28 , DOI: 10.1371/journal.pmed.1003045 Thomas A Willis 1 , Michelle Collinson 2 , Liz Glidewell 3 , Amanda J Farrin 2 , Michael Holland 2 , David Meads 1 , Claire Hulme 4 , Duncan Petty 5 , Sarah Alderson 1 , Suzanne Hartley 2 , Armando Vargas-Palacios 1 , Paul Carder 6 , Stella Johnson 6 , Robbie Foy 1 ,
PLOS Medicine ( IF 15.8 ) Pub Date : 2020-02-28 , DOI: 10.1371/journal.pmed.1003045 Thomas A Willis 1 , Michelle Collinson 2 , Liz Glidewell 3 , Amanda J Farrin 2 , Michael Holland 2 , David Meads 1 , Claire Hulme 4 , Duncan Petty 5 , Sarah Alderson 1 , Suzanne Hartley 2 , Armando Vargas-Palacios 1 , Paul Carder 6 , Stella Johnson 6 , Robbie Foy 1 ,
Affiliation
|
BACKGROUND
In primary care, multiple priorities and system pressures make closing the gap between evidence and practice challenging. Most implementation studies focus on single conditions, limiting generalisability. We compared an adaptable implementation package against an implementation control and assessed effects on adherence to four different evidence-based quality indicators.
METHODS AND FINDINGS
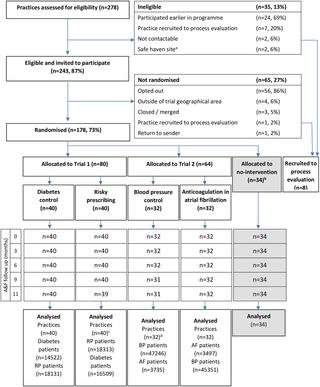
We undertook two parallel, pragmatic cluster-randomised trials using balanced incomplete block designs in general practices in West Yorkshire, England. We used 'opt-out' recruitment, and we randomly assigned practices that did not opt out to an implementation package targeting either diabetes control or risky prescribing (Trial 1); or blood pressure (BP) control or anticoagulation in atrial fibrillation (AF) (Trial 2). Within trials, each arm acted as the implementation control comparison for the other targeted indicator. For example, practices assigned to the diabetes control package acted as the comparison for practices assigned to the risky prescribing package. The implementation package embedded behaviour change techniques within audit and feedback, educational outreach, and computerised support, with content tailored to each indicator. Respective patient-level primary endpoints at 11 months comprised the following: achievement of all recommended levels of haemoglobin A1c (HbA1c), BP, and cholesterol; risky prescribing levels; achievement of recommended BP; and anticoagulation prescribing. Between February and March 2015, we recruited 144 general practices collectively serving over 1 million patients. We stratified computer-generated randomisation by area, list size, and pre-intervention outcome achievement. In April 2015, we randomised 80 practices to Trial 1 (40 per arm) and 64 to Trial 2 (32 per arm). Practices and trial personnel were not blind to allocation. Two practices were lost to follow-up but provided some outcome data. We analysed the intention-to-treat (ITT) population, adjusted for potential confounders at patient level (sex, age) and practice level (list size, locality, pre-intervention achievement against primary outcomes, total quality scores, and levels of patient co-morbidity), and analysed cost-effectiveness. The implementation package reduced risky prescribing (odds ratio [OR] 0.82; 97.5% confidence interval [CI] 0.67-0.99, p = 0.017) with an incremental cost-effectiveness ratio of £1,359 per quality-adjusted life year (QALY), but there was insufficient evidence of effect on other primary endpoints (diabetes control OR 1.03, 97.5% CI 0.89-1.18, p = 0.693; BP control OR 1.05, 97.5% CI 0.96-1.16, p = 0.215; anticoagulation prescribing OR 0.90, 97.5% CI 0.75-1.09, p = 0.214). No statistically significant effects were observed in any secondary outcome except for reduced co-prescription of aspirin and clopidogrel without gastro-protection in patients aged 65 and over (adjusted OR 0.62; 97.5% CI 0.39-0.99; p = 0.021). Main study limitations concern our inability to make any inferences about the relative effects of individual intervention components, given the multifaceted nature of the implementation package, and that the composite endpoint for diabetes control may have been too challenging to achieve.
CONCLUSIONS
In this study, we observed that a multifaceted implementation package was clinically and cost-effective for targeting prescribing behaviours within the control of clinicians but not for more complex behaviours that also required patient engagement.
TRIAL REGISTRATION
The study is registered with the ISRCTN registry (ISRCTN91989345).
中文翻译:

针对初级保健中基于证据的指标的适应性实施包:实用的集群随机评估。
背景技术在初级保健中,多重优先权和系统压力使得弥合证据与实践之间的鸿沟具有挑战性。大多数实施研究只关注单个条件,从而限制了可推广性。我们将适应性强的实施方案包与实施控制措施进行了比较,并评估了遵守四个不同的循证质量指标对遵守情况的影响。方法和结果我们在英国西约克郡进行了两项平行的,实用的,采用均衡不完全区组设计的实用随机试验。我们采用“退出”招募方式,我们随机分配了没有退出的实践,以针对糖尿病控制或危险处方的实施方案(试验1);或控制房颤(AF)中的血压(BP)或抗凝(试验2)。在试验中 每个部门都充当其他目标指标的实施控制比较。例如,分配给糖尿病控制方案的实践与分配给危险处方方案的实践进行比较。该实施程序包将行为更改技术嵌入了审核和反馈,教育范围和计算机化支持内,并针对每个指标量身定制了内容。在11个月时,各个患者的主要终点指标包括:达到所有推荐的血红蛋白A1c(HbA1c),BP和胆固醇水平;危险的处方水平;达到推荐的血压;和抗凝处方。在2015年2月至2015年3月之间,我们共招募了144种常规做法,为超过100万患者提供服务。我们按面积,列表大小,和干预前的成果。2015年4月,我们将80个练习随机分为第1次试验(每组40个)和64个第2次试验(每组32个)。实践和审判人员不盲目分配。随访中丢失了两种做法,但提供了一些结果数据。我们分析了意向性治疗(ITT)人群,并根据患者水平(性别,年龄)和实践水平(列表大小,位置,干预前相对主要结果,总质量得分和患者水平)的潜在混杂因素进行了调整合并症),并分析了成本效益。该实施包减少了风险处方(赔率[OR] 0.82; 97.5%置信区间[CI] 0.67-0.99,p = 0.017),每质量调整生命年(QALY)的成本效益比增加了1,359英镑,但没有足够的证据证明对其他主要终点有影响(糖尿病对照OR 1.03,97.5%CI 0.89-1.18,p = 0.693; BP对照OR 1.05,97.5%CI 0.96-1.16,p = 0.215;抗凝处方OR 0.90,97.5 %CI 0.75-1.09,p = 0.214)。在65岁及以上的患者中,除了阿司匹林和氯吡格雷的联合处方减少且无胃保护外,在任何次要结局中均未观察到统计学上的显着影响(校正OR 0.62; 97.5%CI 0.39-0.99; p = 0.021)。鉴于实施方案的多方面性质,主要的研究局限性涉及我们无法对单个干预成分的相对作用做出任何推断,而且糖尿病控制的综合终点可能难以实现。结论在这项研究中,我们观察到,针对目标是在临床医生控制范围内的处方行为,但针对需要患者参与的更复杂的行为,多方面的实施方案在临床上和成本效益方面都非常有效。试验注册本研究已在ISRCTN注册中心(ISRCTN91989345)注册。
更新日期:2020-03-02
中文翻译:

针对初级保健中基于证据的指标的适应性实施包:实用的集群随机评估。
背景技术在初级保健中,多重优先权和系统压力使得弥合证据与实践之间的鸿沟具有挑战性。大多数实施研究只关注单个条件,从而限制了可推广性。我们将适应性强的实施方案包与实施控制措施进行了比较,并评估了遵守四个不同的循证质量指标对遵守情况的影响。方法和结果我们在英国西约克郡进行了两项平行的,实用的,采用均衡不完全区组设计的实用随机试验。我们采用“退出”招募方式,我们随机分配了没有退出的实践,以针对糖尿病控制或危险处方的实施方案(试验1);或控制房颤(AF)中的血压(BP)或抗凝(试验2)。在试验中 每个部门都充当其他目标指标的实施控制比较。例如,分配给糖尿病控制方案的实践与分配给危险处方方案的实践进行比较。该实施程序包将行为更改技术嵌入了审核和反馈,教育范围和计算机化支持内,并针对每个指标量身定制了内容。在11个月时,各个患者的主要终点指标包括:达到所有推荐的血红蛋白A1c(HbA1c),BP和胆固醇水平;危险的处方水平;达到推荐的血压;和抗凝处方。在2015年2月至2015年3月之间,我们共招募了144种常规做法,为超过100万患者提供服务。我们按面积,列表大小,和干预前的成果。2015年4月,我们将80个练习随机分为第1次试验(每组40个)和64个第2次试验(每组32个)。实践和审判人员不盲目分配。随访中丢失了两种做法,但提供了一些结果数据。我们分析了意向性治疗(ITT)人群,并根据患者水平(性别,年龄)和实践水平(列表大小,位置,干预前相对主要结果,总质量得分和患者水平)的潜在混杂因素进行了调整合并症),并分析了成本效益。该实施包减少了风险处方(赔率[OR] 0.82; 97.5%置信区间[CI] 0.67-0.99,p = 0.017),每质量调整生命年(QALY)的成本效益比增加了1,359英镑,但没有足够的证据证明对其他主要终点有影响(糖尿病对照OR 1.03,97.5%CI 0.89-1.18,p = 0.693; BP对照OR 1.05,97.5%CI 0.96-1.16,p = 0.215;抗凝处方OR 0.90,97.5 %CI 0.75-1.09,p = 0.214)。在65岁及以上的患者中,除了阿司匹林和氯吡格雷的联合处方减少且无胃保护外,在任何次要结局中均未观察到统计学上的显着影响(校正OR 0.62; 97.5%CI 0.39-0.99; p = 0.021)。鉴于实施方案的多方面性质,主要的研究局限性涉及我们无法对单个干预成分的相对作用做出任何推断,而且糖尿病控制的综合终点可能难以实现。结论在这项研究中,我们观察到,针对目标是在临床医生控制范围内的处方行为,但针对需要患者参与的更复杂的行为,多方面的实施方案在临床上和成本效益方面都非常有效。试验注册本研究已在ISRCTN注册中心(ISRCTN91989345)注册。



























 京公网安备 11010802027423号
京公网安备 11010802027423号