当前位置:
X-MOL 学术
›
Mucosal Immunol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spatial proteomics revealed a CX3CL1-dependent crosstalk between the urothelium and relocated macrophages through IL-6 during an acute bacterial infection in the urinary bladder.
Mucosal Immunology ( IF 7.9 ) Pub Date : 2020-02-28 , DOI: 10.1038/s41385-020-0269-7 Jenny Bottek 1 , Camille Soun 1 , Julia K Lill 1 , Akanksha Dixit 1 , Stephanie Thiebes 1 , Anna-Lena Beerlage 1 , Marius Horstmann 1 , Annett Urbanek 2 , Heike Heuer 3 , Julian Uszkoreit 4 , Martin Eisenacher 4 , Thilo Bracht 4 , Barbara Sitek 4 , Franziska Hoffmann 5 , Nirojah Vijitha 1 , Ferdinand von Eggeling 5 , Daniel R Engel 1
Mucosal Immunology ( IF 7.9 ) Pub Date : 2020-02-28 , DOI: 10.1038/s41385-020-0269-7 Jenny Bottek 1 , Camille Soun 1 , Julia K Lill 1 , Akanksha Dixit 1 , Stephanie Thiebes 1 , Anna-Lena Beerlage 1 , Marius Horstmann 1 , Annett Urbanek 2 , Heike Heuer 3 , Julian Uszkoreit 4 , Martin Eisenacher 4 , Thilo Bracht 4 , Barbara Sitek 4 , Franziska Hoffmann 5 , Nirojah Vijitha 1 , Ferdinand von Eggeling 5 , Daniel R Engel 1
Affiliation
|
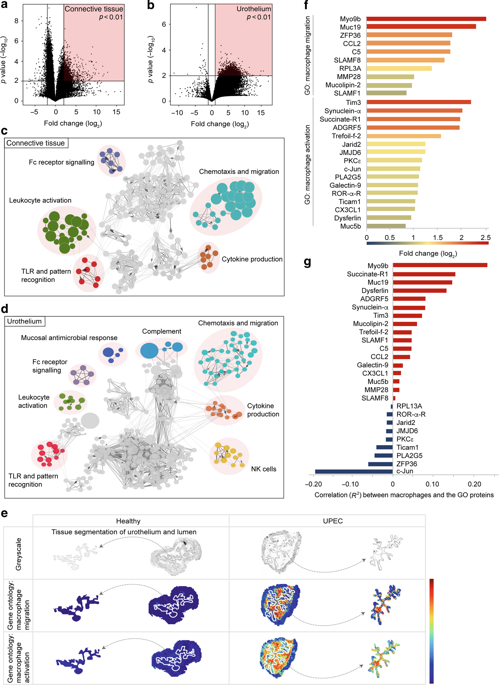
The urothelium of the urinary bladder represents the first line of defense. However, uropathogenic E. coli (UPEC) damage the urothelium and cause acute bacterial infection. Here, we demonstrate the crosstalk between macrophages and the urothelium stimulating macrophage migration into the urothelium. Using spatial proteomics by MALDI-MSI and LC-MS/MS, a novel algorithm revealed the spatial activation and migration of macrophages. Analysis of the spatial proteome unravelled the coexpression of Myo9b and F4/80 in the infected urothelium, indicating that macrophages have entered the urothelium upon infection. Immunofluorescence microscopy additionally indicated that intraurothelial macrophages phagocytosed UPEC and eliminated neutrophils. Further analysis of the spatial proteome by MALDI-MSI showed strong expression of IL-6 in the urothelium and local inhibition of this molecule reduced macrophage migration into the urothelium and aggravated the infection. After IL-6 inhibition, the expression of matrix metalloproteinases and chemokines, such as CX3CL1 was reduced in the urothelium. Accordingly, macrophage migration into the urothelium was diminished in the absence of CX3CL1 signaling in Cx3cr1gfp/gfp mice. Conclusively, this study describes the crosstalk between the infected urothelium and macrophages through IL-6-induced CX3CL1 expression. Such crosstalk facilitates the relocation of macrophages into the urothelium and reduces bacterial burden in the urinary bladder.
中文翻译:

空间蛋白质组学揭示了膀胱急性细菌感染期间尿路上皮和通过 IL-6 重新定位的巨噬细胞之间的 CX3CL1 依赖性串扰。
膀胱的尿路上皮代表第一道防线。然而,尿路致病性大肠杆菌 (UPEC) 会破坏尿路上皮并引起急性细菌感染。在这里,我们展示了巨噬细胞和尿路上皮刺激巨噬细胞迁移到尿路上皮之间的串扰。使用 MALDI-MSI 和 LC-MS/MS 的空间蛋白质组学,一种新算法揭示了巨噬细胞的空间激活和迁移。空间蛋白质组分析揭示了 Myo9b 和 F4/80 在受感染的尿路上皮中的共表达,表明巨噬细胞在感染后进入了尿路上皮。免疫荧光显微镜还表明,尿路上皮内巨噬细胞吞噬 UPEC 并消除中性粒细胞。通过 MALDI-MSI 对空间蛋白质组的进一步分析显示 IL-6 在尿路上皮中的强表达和该分子的局部抑制减少了巨噬细胞向尿路上皮的迁移并加重了感染。抑制 IL-6 后,尿路上皮中基质金属蛋白酶和趋化因子(如 CX3CL1)的表达减少。因此,在 Cx3cr1gfp/gfp 小鼠中,在没有 CX3CL1 信号传导的情况下,巨噬细胞向尿路上皮的迁移减少。最后,本研究通过 IL-6 诱导的 CX3CL1 表达描述了感染的尿路上皮和巨噬细胞之间的串扰。这种串扰有助于巨噬细胞重新定位到尿路上皮并减少膀胱中的细菌负荷。抑制 IL-6 后,尿路上皮中基质金属蛋白酶和趋化因子(如 CX3CL1)的表达减少。因此,在 Cx3cr1gfp/gfp 小鼠中,在没有 CX3CL1 信号传导的情况下,巨噬细胞向尿路上皮的迁移减少。最后,本研究通过 IL-6 诱导的 CX3CL1 表达描述了感染的尿路上皮和巨噬细胞之间的串扰。这种串扰有助于巨噬细胞重新定位到尿路上皮并减少膀胱中的细菌负荷。抑制 IL-6 后,尿路上皮中基质金属蛋白酶和趋化因子(如 CX3CL1)的表达减少。因此,在 Cx3cr1gfp/gfp 小鼠中,在没有 CX3CL1 信号传导的情况下,巨噬细胞向尿路上皮的迁移减少。最后,本研究通过 IL-6 诱导的 CX3CL1 表达描述了感染的尿路上皮和巨噬细胞之间的串扰。这种串扰有助于巨噬细胞重新定位到尿路上皮并减少膀胱中的细菌负荷。本研究通过 IL-6 诱导的 CX3CL1 表达描述了感染的尿路上皮和巨噬细胞之间的串扰。这种串扰有助于巨噬细胞重新定位到尿路上皮并减少膀胱中的细菌负荷。本研究通过 IL-6 诱导的 CX3CL1 表达描述了感染的尿路上皮和巨噬细胞之间的串扰。这种串扰有助于巨噬细胞重新定位到尿路上皮并减少膀胱中的细菌负荷。
更新日期:2020-02-28
中文翻译:

空间蛋白质组学揭示了膀胱急性细菌感染期间尿路上皮和通过 IL-6 重新定位的巨噬细胞之间的 CX3CL1 依赖性串扰。
膀胱的尿路上皮代表第一道防线。然而,尿路致病性大肠杆菌 (UPEC) 会破坏尿路上皮并引起急性细菌感染。在这里,我们展示了巨噬细胞和尿路上皮刺激巨噬细胞迁移到尿路上皮之间的串扰。使用 MALDI-MSI 和 LC-MS/MS 的空间蛋白质组学,一种新算法揭示了巨噬细胞的空间激活和迁移。空间蛋白质组分析揭示了 Myo9b 和 F4/80 在受感染的尿路上皮中的共表达,表明巨噬细胞在感染后进入了尿路上皮。免疫荧光显微镜还表明,尿路上皮内巨噬细胞吞噬 UPEC 并消除中性粒细胞。通过 MALDI-MSI 对空间蛋白质组的进一步分析显示 IL-6 在尿路上皮中的强表达和该分子的局部抑制减少了巨噬细胞向尿路上皮的迁移并加重了感染。抑制 IL-6 后,尿路上皮中基质金属蛋白酶和趋化因子(如 CX3CL1)的表达减少。因此,在 Cx3cr1gfp/gfp 小鼠中,在没有 CX3CL1 信号传导的情况下,巨噬细胞向尿路上皮的迁移减少。最后,本研究通过 IL-6 诱导的 CX3CL1 表达描述了感染的尿路上皮和巨噬细胞之间的串扰。这种串扰有助于巨噬细胞重新定位到尿路上皮并减少膀胱中的细菌负荷。抑制 IL-6 后,尿路上皮中基质金属蛋白酶和趋化因子(如 CX3CL1)的表达减少。因此,在 Cx3cr1gfp/gfp 小鼠中,在没有 CX3CL1 信号传导的情况下,巨噬细胞向尿路上皮的迁移减少。最后,本研究通过 IL-6 诱导的 CX3CL1 表达描述了感染的尿路上皮和巨噬细胞之间的串扰。这种串扰有助于巨噬细胞重新定位到尿路上皮并减少膀胱中的细菌负荷。抑制 IL-6 后,尿路上皮中基质金属蛋白酶和趋化因子(如 CX3CL1)的表达减少。因此,在 Cx3cr1gfp/gfp 小鼠中,在没有 CX3CL1 信号传导的情况下,巨噬细胞向尿路上皮的迁移减少。最后,本研究通过 IL-6 诱导的 CX3CL1 表达描述了感染的尿路上皮和巨噬细胞之间的串扰。这种串扰有助于巨噬细胞重新定位到尿路上皮并减少膀胱中的细菌负荷。本研究通过 IL-6 诱导的 CX3CL1 表达描述了感染的尿路上皮和巨噬细胞之间的串扰。这种串扰有助于巨噬细胞重新定位到尿路上皮并减少膀胱中的细菌负荷。本研究通过 IL-6 诱导的 CX3CL1 表达描述了感染的尿路上皮和巨噬细胞之间的串扰。这种串扰有助于巨噬细胞重新定位到尿路上皮并减少膀胱中的细菌负荷。











































 京公网安备 11010802027423号
京公网安备 11010802027423号