Oncogene ( IF 8 ) Pub Date : 2020-03-02 , DOI: 10.1038/s41388-020-1209-4 Enric Redondo Monte 1, 2, 3 , Anja Wilding 1, 2, 3 , Georg Leubolt 1, 2, 3 , Paul Kerbs 1, 2, 3 , Johannes W Bagnoli 4 , Luise Hartmann 1, 2, 3 , Wolfgang Hiddemann 1, 2, 3 , Linping Chen-Wichmann 5 , Stefan Krebs 6 , Helmut Blum 6 , Monica Cusan 1 , Binje Vick 7 , Irmela Jeremias 7 , Wolfgang Enard 4 , Sebastian Theurich 1, 8 , Christian Wichmann 5 , Philipp A Greif 1, 2, 3
|
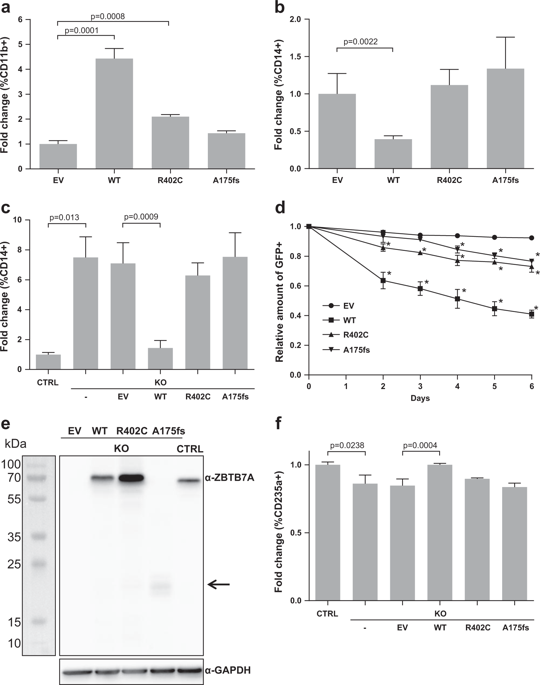
ZBTB7A is frequently mutated in acute myeloid leukemia (AML) with t(8;21) translocation. However, the oncogenic collaboration between mutated ZBTB7A and the RUNX1–RUNX1T1 fusion gene in AML t(8;21) remains unclear. Here, we investigate the role of ZBTB7A and its mutations in the context of normal and malignant hematopoiesis. We demonstrate that clinically relevant ZBTB7A mutations in AML t(8;21) lead to loss of function and result in perturbed myeloid differentiation with block of the granulocytic lineage in favor of monocytic commitment. In addition, loss of ZBTB7A increases glycolysis and hence sensitizes leukemic blasts to metabolic inhibition with 2-deoxy-d-glucose. We observed that ectopic expression of wild-type ZBTB7A prevents RUNX1-RUNX1T1-mediated clonal expansion of human CD34+ cells, whereas the outgrowth of progenitors is enabled by ZBTB7A mutation. Finally, ZBTB7A expression in t(8;21) cells lead to a cell cycle arrest that could be mimicked by inhibition of glycolysis. Our findings suggest that loss of ZBTB7A may facilitate the onset of AML t(8;21), and that RUNX1-RUNX1T1-rearranged leukemia might be treated with glycolytic inhibitors.
中文翻译:

ZBTB7A 阻止人类造血干细胞和祖细胞的 RUNX1-RUNX1T1 依赖性克隆扩增
ZBTB7A 在急性髓性白血病 (AML) 中经常发生突变,并伴有 t(8;21) 易位。然而,突变的 ZBTB7A 与 AML t(8;21) 中的 RUNX1-RUNX1T1 融合基因之间的致癌协作仍不清楚。在这里,我们研究了 ZBTB7A 及其突变在正常和恶性造血背景下的作用。我们证明 AML t(8;21) 中临床相关的 ZBTB7A 突变导致功能丧失,并导致粒细胞谱系受阻导致骨髓分化受干扰,有利于单核细胞承诺。此外,ZBTB7A 的缺失会增加糖酵解,从而使白血病原始细胞对 2-脱氧-d的代谢抑制敏感。-葡萄糖。我们观察到野生型 ZBTB7A 的异位表达阻止了 RUNX1-RUNX1T1 介导的人类 CD34+ 细胞的克隆扩增,而 ZBTB7A 突变使祖细胞的生长成为可能。最后,t(8;21) 细胞中的 ZBTB7A 表达导致细胞周期停滞,这可以通过抑制糖酵解来模拟。我们的研究结果表明,ZBTB7A 的缺失可能会促进 AML t(8;21) 的发作,并且 RUNX1-RUNX1T1 重排的白血病可能会用糖酵解抑制剂治疗。



























 京公网安备 11010802027423号
京公网安备 11010802027423号