当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Terminal charges modulate the pore forming activity of cationic amphipathic helices.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-02-29 , DOI: 10.1016/j.bbamem.2020.183243 Erik Strandberg 1 , David Bentz 2 , Parvesh Wadhwani 1 , Jochen Bürck 1 , Anne S Ulrich 3
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-02-29 , DOI: 10.1016/j.bbamem.2020.183243 Erik Strandberg 1 , David Bentz 2 , Parvesh Wadhwani 1 , Jochen Bürck 1 , Anne S Ulrich 3
Affiliation
|
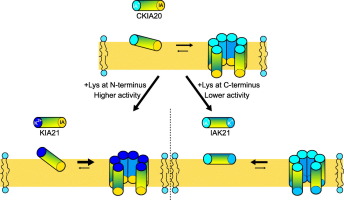
KIA peptides are a series of designer-made cationic amphipathic α-helical antimicrobial peptides of different lengths, based on the repetitive sequence [KIAGKIA]. They can form toroidal pores in membranes, wherein the helices are aligned in a transmembrane orientation. Solid-state 15N NMR is used here to differentiate between the surface-bound and transmembrane states. We find that the pore-forming activity increases when the peptides carry a positive charge (Lys residue) at the N-terminus, compared to a hydrophobic Ile-Ala N-terminal motif. In contrast, a positive charge at the C-terminus gives a lower membrane activity compared to C-terminal Ile-Ala. For peptides with otherwise identical sequence, a more than ten-fold difference in vesicle leakage can be observed, depending on which terminus carries the charge. This difference is attributed to a shift in the equilibrium between peptide helices oriented on the membrane surface and those inserted into the membrane in a pore-forming state. We show that the 3D hydrophobic moment can be used to predict which peptide sequence is more prone to form pores and will thereby show a higher membranolytic activity.
中文翻译:

末端电荷调节阳离子两亲性螺旋的孔形成活性。
KIA肽是一系列基于重复序列[KIAGKIA]的不同长度的设计师设计的阳离子两亲性α-螺旋抗菌肽。它们可以在膜中形成环形孔,其中螺旋以跨膜取向排列。此处使用固态15N NMR区分表面结合态和跨膜态。我们发现,与疏水性Ile-Ala N端基序相比,当肽在N端带有正电荷(Lys残基)时,成孔活性增加。相反,与C端Ile-Ala相比,C端的正电荷具有较低的膜活性。对于其他序列相同的肽,根据哪个末端带电荷,可以观察到小泡泄漏的差异超过十倍。该差异归因于定向在膜表面上的肽螺旋与以孔形成状态插入膜中的那些螺旋之间的平衡的偏移。我们表明3D疏水矩可用于预测哪个肽序列更易于形成孔,从而显示出更高的膜分解活性。
更新日期:2020-03-02
中文翻译:

末端电荷调节阳离子两亲性螺旋的孔形成活性。
KIA肽是一系列基于重复序列[KIAGKIA]的不同长度的设计师设计的阳离子两亲性α-螺旋抗菌肽。它们可以在膜中形成环形孔,其中螺旋以跨膜取向排列。此处使用固态15N NMR区分表面结合态和跨膜态。我们发现,与疏水性Ile-Ala N端基序相比,当肽在N端带有正电荷(Lys残基)时,成孔活性增加。相反,与C端Ile-Ala相比,C端的正电荷具有较低的膜活性。对于其他序列相同的肽,根据哪个末端带电荷,可以观察到小泡泄漏的差异超过十倍。该差异归因于定向在膜表面上的肽螺旋与以孔形成状态插入膜中的那些螺旋之间的平衡的偏移。我们表明3D疏水矩可用于预测哪个肽序列更易于形成孔,从而显示出更高的膜分解活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号