当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation of mouse NBCe1-B by Xenopus laevis and mouse IRBITs: Role of the variable Nt appendage of IRBITs.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-02-28 , DOI: 10.1016/j.bbamem.2020.183240 Meng Wang 1 , Han Wu 1 , Ying Liu 1 , Li-Ming Chen 1
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-02-28 , DOI: 10.1016/j.bbamem.2020.183240 Meng Wang 1 , Han Wu 1 , Ying Liu 1 , Li-Ming Chen 1
Affiliation
|
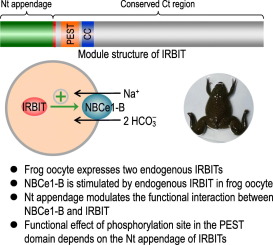
The IP3 receptor binding protein released with inositol 1,4,5-trisphosphate (IRBIT) plays important roles in the regulation of intracellular Ca2+ signaling and intracellular pH. The mammals express two IRBIT paralogs, i.e., IRBIT1 (encoded by AHCYL1) and IRBIT2 (encoded by AHCYL2). The clawed frog Xenopus laevis oocyte is widely used for biophysical studies on ion channels and transporters. It remains unknown whether endogenous IRBIT is expressed in Xenopus oocytes. Here, we cloned from frog oocyte irbit2.L and irbit2.S, orthologs of mammalian IRBIT2. When over-expressed, the frog IRBITs powerfully stimulate the electrogenic Na+/HCO3- cotransporter NBCe1-B as mouse IRBIT2-V2 does. Expression of an isolated Nt fragment of NBCe1-B containing the IRBIT-binding domain greatly decreases NBCe1-B activity in oocytes, suggesting that the basal activity of NBCe1-B contains a large component derived from the stimulation by endogenous frog IRBIT. The frog IRBITs are highly homologous to the mammalian ones in the carboxyl-terminal region, but varies greatly in the amino-terminal (Nt) appendage. Interestingly, truncation study showed that the Nt appendage of IRBIT1 and the long Nt appendage of IRBIT2-V2 modestly enhance, whereas the short Nt appendage of IRBIT2-V4 greatly inhibits the functional interaction between IRBIT and NBCe1-B. Finally, Ala-substitution of Ser68, a key phosphorylation site in the PEST domain of IRBIT, causes distinct functional consequences depending on the structural context of the Nt appendage in different IRBIT isoforms. We conclude that the Nt appendage of IRBITs is not necessary for, but plays an important regulatory role in the functional interaction between IRBIT and NBCe1-B.
中文翻译:

非洲爪蟾和小鼠IRBITs对小鼠NBCe1-B的激活:IRBITs的可变Nt附件的作用。
肌醇1,4,5-三磷酸酯(IRBIT)释放的IP3受体结合蛋白在调节细胞内Ca2 +信号传导和细胞内pH中起重要作用。哺乳动物表达了两个IRBIT同源物,即IRBIT1(由AHCYL1编码)和IRBIT2(由AHCYL2编码)。爪蛙非洲爪蟾卵母细胞被广泛用于离子通道和转运蛋白的生物物理研究。爪蟾卵母细胞中是否表达内源性IRBIT仍是未知的。在这里,我们从青蛙卵母细胞irbit2.L和irbit2.S(哺乳动物IRBIT2的直系同源物中)克隆。过度表达时,青蛙IRBITs可以像小鼠IRBIT2-V2一样,强烈刺激电致Na + / HCO3-共转运蛋白NBCe1-B。含有IRBIT结合域的NBCe1-B分离Nt片段的表达大大降低了卵母细胞中NBCe1-B的活性,提示NBCe1-B的基础活性包含很大一部分来自内源性青蛙IRBIT的刺激。青蛙的IRBITs在羧基末端区域与哺乳动物的IRBITs具有高度同源性,但在氨基末端(Nt)附肢中差异很大。有趣的是,截短研究表明,IRBIT1的Nt附件和IRBIT2-V2的长Nt附件适度增强,而IRBIT2-V4的短Nt附件极大地抑制了IRBIT和NBCe1-B之间的功能相互作用。最后,Ser68的Ala取代是IRBIT的PEST域中的一个关键磷酸化位点,根据不同IRBIT亚型中Nt附肢的结构背景,会引起不同的功能性后果。我们得出结论,对于,
更新日期:2020-03-02
中文翻译:

非洲爪蟾和小鼠IRBITs对小鼠NBCe1-B的激活:IRBITs的可变Nt附件的作用。
肌醇1,4,5-三磷酸酯(IRBIT)释放的IP3受体结合蛋白在调节细胞内Ca2 +信号传导和细胞内pH中起重要作用。哺乳动物表达了两个IRBIT同源物,即IRBIT1(由AHCYL1编码)和IRBIT2(由AHCYL2编码)。爪蛙非洲爪蟾卵母细胞被广泛用于离子通道和转运蛋白的生物物理研究。爪蟾卵母细胞中是否表达内源性IRBIT仍是未知的。在这里,我们从青蛙卵母细胞irbit2.L和irbit2.S(哺乳动物IRBIT2的直系同源物中)克隆。过度表达时,青蛙IRBITs可以像小鼠IRBIT2-V2一样,强烈刺激电致Na + / HCO3-共转运蛋白NBCe1-B。含有IRBIT结合域的NBCe1-B分离Nt片段的表达大大降低了卵母细胞中NBCe1-B的活性,提示NBCe1-B的基础活性包含很大一部分来自内源性青蛙IRBIT的刺激。青蛙的IRBITs在羧基末端区域与哺乳动物的IRBITs具有高度同源性,但在氨基末端(Nt)附肢中差异很大。有趣的是,截短研究表明,IRBIT1的Nt附件和IRBIT2-V2的长Nt附件适度增强,而IRBIT2-V4的短Nt附件极大地抑制了IRBIT和NBCe1-B之间的功能相互作用。最后,Ser68的Ala取代是IRBIT的PEST域中的一个关键磷酸化位点,根据不同IRBIT亚型中Nt附肢的结构背景,会引起不同的功能性后果。我们得出结论,对于,











































 京公网安备 11010802027423号
京公网安备 11010802027423号