European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2020-02-29 , DOI: 10.1016/j.ejmech.2020.112193 Hui-Ju Tseng , Mei-Hsiang Lin , Young-Ji Shiao , Ying-Chen Yang , Jung-Chun Chu , Chun-Yung Chen , Yi-Ying Chen , Tony Eight Lin , Chih-Jou Su , Shiow-Lin Pan , Liang-Chieh Chen , Chen-Yu Wang , Kai-Cheng Hsu , Wei-Jan Huang
|
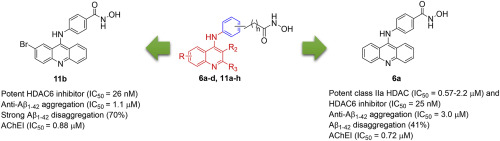
Multitarget agents simultaneously trigger molecules in functionally complementary pathways, and are therefore considered to have potential in effectively treating Alzheimer's disease (AD), which has a complex pathogenetic mechanism. In this study, the HDAC inhibitor core is incorporated into the acetylcholine esterase (ACE) inhibitor acridine–derived moiety. Some resulting compounds exhibited higher class IIa HDAC (4, 5, 7, and 9)- and class IIb HDAC6-inhibiting activity than did the reference SAHA as a pan-HDAC inhibitor in clinical practice. One of these compounds, 11b, displayed greater selectivity toward HDAC6 than other isoform enzymes. In contrast, the activity of compound 6a was selective toward class IIa HDAC and HDAC6. These two compounds exhibited strong activity against Aβ-aggregation as well as significantly disrupted Aβ-oligomer. Additionally, 11b and 6a strongly inhibited AChE. These experimental findings demonstrate that compounds 11b and 6a are multiple HDAC-Aβ-aggregation-AChE inhibitors. Notably, they can enhance neurite outgrowth, but with no significant neurotoxicity. Further biological evaluation revealed the various cellular effects of multitarget compounds 11b and 6a, which have the potential to treat AD.
中文翻译:

a啶基组蛋白脱乙酰基酶抑制剂作为抗阿尔茨海默氏病多靶标药物的合成及生物学评价
多靶点药物同时触发功能互补途径中的分子,因此被认为具有有效治疗阿尔茨海默氏病(AD)的潜力,该病具有复杂的致病机制。在这项研究中,HDAC抑制剂核心被掺入乙酰胆碱酯酶(ACE)抑制剂a啶衍生的部分。在临床实践中,某些化合物显示出比参考SAHA作为泛HDAC抑制剂更高的IIa类HDAC(4、5、7和9)和IIb类HDAC6抑制活性。这些化合物之一11b对HDAC6的选择性高于其他同工酶。相反,化合物6a的活性对IIa类HDAC和HDAC6有选择性。这两种化合物显示出强大的抗Aβ聚集活性以及显着破坏的Aβ低聚物。另外,11b和6a强烈抑制AChE。这些实验结果表明,化合物11b和6a是多种HDAC-Aβ-聚集-AChE抑制剂。值得注意的是,它们可以增强神经突的生长,但没有明显的神经毒性。进一步的生物学评估揭示了多靶化合物11b和6a的各种细胞效应,它们具有治疗AD的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号