当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Excitation energies expressed as orbital energies of Kohn-Sham density functional theory with long-range corrected functionals
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2020-02-28 , DOI: 10.1002/jcc.26181 Kimihiko Hirao 1, 2 , Bun Chan 3 , Jong-Won Song 4 , Kamala Bhattarai 4 , Subrata Tewary 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2020-02-28 , DOI: 10.1002/jcc.26181 Kimihiko Hirao 1, 2 , Bun Chan 3 , Jong-Won Song 4 , Kamala Bhattarai 4 , Subrata Tewary 1
Affiliation
|
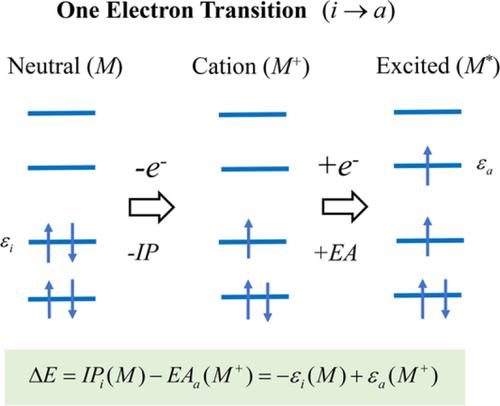
A new simple and conceptual theoretical scheme is proposed for estimating one‐electron excitation energies using Kohn–Sham (KS) solutions. One‐electron transitions that are dominated by the promotion from one initially occupied orbital to one unoccupied orbital of a molecular system can be expressed in a two‐step process, ionization, and electron attachment. KS with long‐range corrected (LC) functionals satisfies Janak's theorem and LC total energy varies almost linearly as a function of its fractional occupation number between the integer electron points. Thus, LC reproduces ionization energies (IPs) and electron affinities (EAs) with high accuracy and one‐electron excitation energies are expressed as the difference between the occupied orbital energy of a neutral molecule and the corresponding unoccupied orbital energy of its cation. Two such expressions can be used, with one employing the orbital energies for the neutral and cationic systems, while the other utilizes orbital energies of just the cation. Because the EA of a molecule is the IP of its anion, if we utilize this identity, the two expressions coincide and give the same excitation energies. Reasonable results are obtained for valence and core excitations using only orbital energies.
中文翻译:

激发能量表示为具有长程校正泛函的 Kohn-Sham 密度泛函理论的轨道能量
提出了一种新的简单且概念化的理论方案,用于使用 Kohn-Sham (KS) 解决方案估算单电子激发能量。由分子系统的一个最初占据轨道到一个未占据轨道的促进主导的单电子跃迁可以用两步过程、电离和电子附着来表达。具有长程校正 (LC) 泛函的 KS 满足 Janak 定理,并且 LC 总能量几乎呈线性变化,作为其整数电子点之间的分数占有数的函数。因此,LC 以高精度再现电离能 (IP) 和电子亲和力 (EA),单电子激发能表示为中性分子的占据轨道能量与其相应的阳离子未占据轨道能量之间的差值。可以使用两种这样的表达式,一种使用中性和阳离子系统的轨道能量,而另一种仅使用阳离子的轨道能量。因为分子的 EA 是其阴离子的 IP,如果我们利用这个身份,这两个表达式会重合并给出相同的激发能。仅使用轨道能量即可获得价态和核心激发的合理结果。
更新日期:2020-02-28
中文翻译:

激发能量表示为具有长程校正泛函的 Kohn-Sham 密度泛函理论的轨道能量
提出了一种新的简单且概念化的理论方案,用于使用 Kohn-Sham (KS) 解决方案估算单电子激发能量。由分子系统的一个最初占据轨道到一个未占据轨道的促进主导的单电子跃迁可以用两步过程、电离和电子附着来表达。具有长程校正 (LC) 泛函的 KS 满足 Janak 定理,并且 LC 总能量几乎呈线性变化,作为其整数电子点之间的分数占有数的函数。因此,LC 以高精度再现电离能 (IP) 和电子亲和力 (EA),单电子激发能表示为中性分子的占据轨道能量与其相应的阳离子未占据轨道能量之间的差值。可以使用两种这样的表达式,一种使用中性和阳离子系统的轨道能量,而另一种仅使用阳离子的轨道能量。因为分子的 EA 是其阴离子的 IP,如果我们利用这个身份,这两个表达式会重合并给出相同的激发能。仅使用轨道能量即可获得价态和核心激发的合理结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号