当前位置:
X-MOL 学术
›
ChemCatChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure and activity of supported bimetallic NiPd nanoparticles: influence of preparation method on CO2 reduction
ChemCatChem ( IF 3.8 ) Pub Date : 2020-02-27 , DOI: 10.1002/cctc.201902329 Adriano H. Braga 1 , Natália J. S. Costa 1 , Karine Philippot 2 , Renato V. Gonçalves 3 , János Szanyi 4 , Liane M. Rossi 1
ChemCatChem ( IF 3.8 ) Pub Date : 2020-02-27 , DOI: 10.1002/cctc.201902329 Adriano H. Braga 1 , Natália J. S. Costa 1 , Karine Philippot 2 , Renato V. Gonçalves 3 , János Szanyi 4 , Liane M. Rossi 1
Affiliation
|
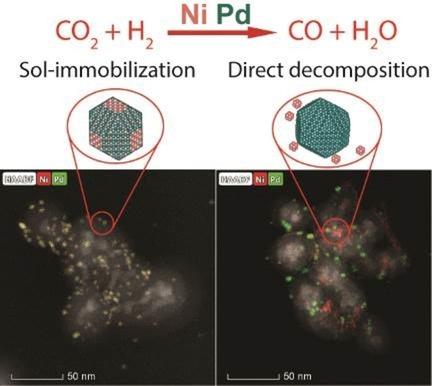
Bimetallic Ni−Pd and monometallic reference catalysts were prepared by decomposing organometallic precursors, Ni(cod)2 and Pd2(dba)3, leading to nanoparticles with sizes ranging from 3 to 6 nm. Two different synthesis procedures were followed: i) solution synthesis using capping ligand (hexadecylamine) followed by impregnation of pre‐formed nanoparticles on SiO2, called Sol‐immobilization (SI); and 2) direct precursor decomposition onto SiO2, without stabilizer, called Direct Decomposition (DD). Samples prepared by SI procedure are alloyed bimetallic nanoparticles, whereas samples obtained by DD one show phase segregation. Interestingly, DD samples show better activity for CO2 hydrogenation into CO (reverse water‐gas shift reaction ‐ RWGS) than SI ones. The best compromise between activity for CO2 activation (at lower temperature) and CO selectivity was achieved with Ni DD and NiPd DD catalysts. Moreover, the addition of palladium increased the concentration of surface undercoordinated sites, which chemisorb CO weakly, thus improving activity and selectivity, in opposition to other samples that chemisorb CO strongly, in multibond configuration. In the presence of Pd, different decomposition rates drives the formation of smaller and more active Ni clusters. The knowledge acquired here on the influence of synthesis conditions on the catalytic properties of Ni−Pd catalysts should guide us to better catalysts for CO2 transformations into valuable products.
中文翻译:

负载型双金属NiPd纳米颗粒的结构和活性:制备方法对CO2还原的影响
通过分解有机金属前驱体Ni(cod)2和Pd 2(dba)3制备双金属Ni-Pd和单金属参比催化剂,得到纳米颗粒,尺寸为3至6 nm。遵循两种不同的合成程序:i)使用封端配体(十六烷基胺)进行溶液合成,然后将预先形成的纳米颗粒浸渍在SiO 2上,称为溶胶固定化(SI);2)在没有稳定剂的情况下将前体直接分解到SiO 2上,称为直接分解(DD)。通过SI方法制备的样品是合金化的双金属纳米颗粒,而通过DD获得的样品显示出相偏析。有趣的是,DD样品显示出更好的CO 2活性氢化成一氧化碳(逆水煤气变换反应-RWGS),比一氧化硅。Ni DD和NiPd DD催化剂在CO 2活化活性(较低温度)和CO选择性之间取得了最佳折衷。此外,钯的加入增加了表面不协调位点的浓度,该位点在化学键作用下弱地吸收了CO,从而与其他化学反应强烈吸收CO的样品相反,提高了活性和选择性。在Pd的存在下,不同的分解速率会驱动更小,更活跃的Ni团簇的形成。此处获得的有关合成条件对Ni-Pd催化剂催化性能影响的知识应指导我们寻找更好的催化剂,以将CO 2转化为有价值的产品。
更新日期:2020-02-27
中文翻译:

负载型双金属NiPd纳米颗粒的结构和活性:制备方法对CO2还原的影响
通过分解有机金属前驱体Ni(cod)2和Pd 2(dba)3制备双金属Ni-Pd和单金属参比催化剂,得到纳米颗粒,尺寸为3至6 nm。遵循两种不同的合成程序:i)使用封端配体(十六烷基胺)进行溶液合成,然后将预先形成的纳米颗粒浸渍在SiO 2上,称为溶胶固定化(SI);2)在没有稳定剂的情况下将前体直接分解到SiO 2上,称为直接分解(DD)。通过SI方法制备的样品是合金化的双金属纳米颗粒,而通过DD获得的样品显示出相偏析。有趣的是,DD样品显示出更好的CO 2活性氢化成一氧化碳(逆水煤气变换反应-RWGS),比一氧化硅。Ni DD和NiPd DD催化剂在CO 2活化活性(较低温度)和CO选择性之间取得了最佳折衷。此外,钯的加入增加了表面不协调位点的浓度,该位点在化学键作用下弱地吸收了CO,从而与其他化学反应强烈吸收CO的样品相反,提高了活性和选择性。在Pd的存在下,不同的分解速率会驱动更小,更活跃的Ni团簇的形成。此处获得的有关合成条件对Ni-Pd催化剂催化性能影响的知识应指导我们寻找更好的催化剂,以将CO 2转化为有价值的产品。










































 京公网安备 11010802027423号
京公网安备 11010802027423号