当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyoxometalate-based crystalline materials as a highly sensitive electrochemical sensor for detecting trace Cr(VI)
Dalton Transactions ( IF 3.5 ) Pub Date : 2020/02/28 , DOI: 10.1039/d0dt00446d Xing Xin 1, 2, 3, 4, 5 , Na Hu 1, 2, 3, 4, 5 , Yuanyuan Ma 1, 2, 3, 4, 5 , Yali Wang 1, 2, 3, 4, 5 , Lin Hou 1, 2, 3, 4, 5 , Heng Zhang 1, 2, 3, 4, 5 , Zhangang Han 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2020/02/28 , DOI: 10.1039/d0dt00446d Xing Xin 1, 2, 3, 4, 5 , Na Hu 1, 2, 3, 4, 5 , Yuanyuan Ma 1, 2, 3, 4, 5 , Yali Wang 1, 2, 3, 4, 5 , Lin Hou 1, 2, 3, 4, 5 , Heng Zhang 1, 2, 3, 4, 5 , Zhangang Han 1, 2, 3, 4, 5
Affiliation
|
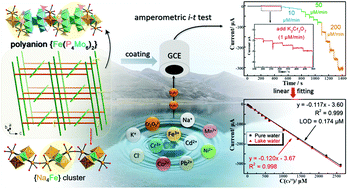
It is crucial to find a convenient and sensitive method for quantitative determination of heavy metal chromium(VI) ions. Developing crystalline materials coupled with polyoxometalates as an electrochemical sensor is a promising approach to address the above issues. Here we reported two reductive polyoxometalate-based crystalline compounds with the formula of (H2bpp)2[Na4Fe(H2O)7][Fe(P4Mo6O31H6)2]·2H2O (1) and (H2bpp)6(bpp)2[Fe(P4Mo6O31H8)2]2·13H2O (2) (bpp = 1,3-bi(4-pyridyl)propane). Structural analysis indicated that both two compounds were composed of inorganic polyanionic clusters and organic protonated bpp cations. The difference lies in the arrangement mode of the inorganic moiety: crystal 1 shows a unique three-dimensional (3-D) inorganic porous skeleton, while crystal 2 consists of isolated 0-D polyanionic clusters. When used as electrochemical sensors in the determination of trace Cr(VI), crystal 1 shows a broad linearity range (2–2610 μM) with a low limit of detection (LOD) of 0.174 μM (9 ppb), which is superior to that of compound 2 (a LOD of 0.33 μM) and meets the standard of Cr(VI) in drinking water set by the WHO (less than 0.962 μM or 50 ppb). Importantly, crystal 1 showed benign selectivity to Cr(VI) in the presence of various heavy metal ions and good reproducibility in a real water sample, which prove its strong anti-interference ability. In addition, experimental results showed that the spatial arrangement of polyanionic clusters could affect the final electrochemical behavior of crystalline materials. This work provides some insights into the design of cost-effective POM-based electrochemical sensors at the molecular level.
中文翻译:

基于多金属氧酸盐的晶体材料,用于检测痕量Cr(VI)的高灵敏度电化学传感器
关键的是要找到定量测定重金属铬(的方便和灵敏的方法VI)离子。开发与多金属氧酸盐偶联的晶体材料作为电化学传感器是解决上述问题的一种有前途的方法。在这里,我们报道了两种还原性多金属氧酸盐基结晶化合物,分子式为(H 2 bpp)2 [Na 4 Fe(H 2 O)7 ] [Fe(P 4 Mo 6 O 31 H 6)2 ]·2H 2 O(1)和(H 2 bpp)6(bpp)2[Fe(P 4 Mo 6 O 31 H 8)2 ] 2 ·13H 2 O(2)(bpp = 1,3-双(4-吡啶基)丙烷)。结构分析表明,这两种化合物均由无机聚阴离子簇和有机质子化的bpp阳离子组成。区别在于无机部分的排列方式:晶体1显示出独特的三维(3-D)无机多孔骨架,而晶体2由孤立的0-D聚阴离子簇组成。当用作测定痕量Cr(VI)的电化学传感器时,晶体1线性范围宽(2–2610μM),检测下限(LOD)为0.174μM(9 ppb),优于化合物2(LOD为0.33μM),符合Cr(VI)在WHO设定的饮用水中(小于0.962μM或50 ppb)。重要的是,晶体1对Cr(VI)存在于各种重金属离子中,并且在真实水样中具有良好的重现性,这证明了其强大的抗干扰能力。此外,实验结果表明,聚阴离子簇的空间排列可能会影响晶体材料的最终电化学行为。这项工作为在分子水平上设计基于成本效益的基于POM的电化学传感器提供了一些见识。
更新日期:2020-04-08
中文翻译:

基于多金属氧酸盐的晶体材料,用于检测痕量Cr(VI)的高灵敏度电化学传感器
关键的是要找到定量测定重金属铬(的方便和灵敏的方法VI)离子。开发与多金属氧酸盐偶联的晶体材料作为电化学传感器是解决上述问题的一种有前途的方法。在这里,我们报道了两种还原性多金属氧酸盐基结晶化合物,分子式为(H 2 bpp)2 [Na 4 Fe(H 2 O)7 ] [Fe(P 4 Mo 6 O 31 H 6)2 ]·2H 2 O(1)和(H 2 bpp)6(bpp)2[Fe(P 4 Mo 6 O 31 H 8)2 ] 2 ·13H 2 O(2)(bpp = 1,3-双(4-吡啶基)丙烷)。结构分析表明,这两种化合物均由无机聚阴离子簇和有机质子化的bpp阳离子组成。区别在于无机部分的排列方式:晶体1显示出独特的三维(3-D)无机多孔骨架,而晶体2由孤立的0-D聚阴离子簇组成。当用作测定痕量Cr(VI)的电化学传感器时,晶体1线性范围宽(2–2610μM),检测下限(LOD)为0.174μM(9 ppb),优于化合物2(LOD为0.33μM),符合Cr(VI)在WHO设定的饮用水中(小于0.962μM或50 ppb)。重要的是,晶体1对Cr(VI)存在于各种重金属离子中,并且在真实水样中具有良好的重现性,这证明了其强大的抗干扰能力。此外,实验结果表明,聚阴离子簇的空间排列可能会影响晶体材料的最终电化学行为。这项工作为在分子水平上设计基于成本效益的基于POM的电化学传感器提供了一些见识。











































 京公网安备 11010802027423号
京公网安备 11010802027423号