当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural evolution of the Ru-bms complex to the real water oxidation catalyst of Ru-bda: the bite angle matters
Dalton Transactions ( IF 3.5 ) Pub Date : 2020/02/28 , DOI: 10.1039/c9dt04693c Jing Yang 1, 2, 3, 4, 5 , Bin Liu 1, 2, 3, 4, 5 , Lele Duan 1, 2, 3, 4, 5
Dalton Transactions ( IF 3.5 ) Pub Date : 2020/02/28 , DOI: 10.1039/c9dt04693c Jing Yang 1, 2, 3, 4, 5 , Bin Liu 1, 2, 3, 4, 5 , Lele Duan 1, 2, 3, 4, 5
Affiliation
|
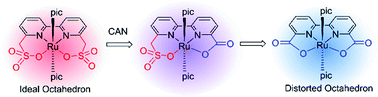
Ru-Based complexes have advanced the study of molecular water oxidation catalysts (WOCs) both in catalysis and mechanism. The electronic effect has always been considered as an essential factor for the catalyst properties while less attention has been focused on the bite angle effect on water oxidation catalysis. The Ru-bda ([Ru(bda)(pic)2]; bda2− = 2,2′-bipyridine-6,6′-dicarboxylate; pic = 4-picoline) catalyst is one of the most active WOCs and it has a largely distorted octahedral configuration with an O–Ru–O bite angle of 123°. Herein, we replaced the carboxylate (–COO−) groups of bda2− with two methylenesulfonate (–CH2SO3−) groups and prepared a negatively charged ligand, bms2− (2,2′-bipyridine-6,6′-dimethanesulfonate), and the Ru-bms complex [Ru(bms)(pic)2]. The O–Ru–O bite angle changed from 123° in Ru-bda to 84° in Ru-bms, leading to a dramatic influence on the catalytic behavior. Systematic analysis of the reaction intermediates suggested that Ru-bms transformed all the way to Ru-bdavia oxidative decomposition under CeIV-driven water oxidation conditions.
中文翻译:

Ru-bms配合物到Ru-bda的真实水氧化催化剂的结构演变:咬合角度很重要
钌基络合物在分子催化和机理研究方面都取得了进展。电子效应一直被认为是催化剂性能的重要因素,而对水氧化催化的咬合角效应的关注则较少。的Ru的BDA(的[Ru(BDA)(PIC)2 ]; BDA 2- = 2,2'-联吡啶-6,6'-二羧酸酯; PIC = 4-甲基吡啶)催化剂是最活跃的WOC之一,它具有严重变形的八面体结构,O–Ru–O咬合角为123°。在此,我们取代羧酸酯(-COO -)BDA的基团2-具有两个亚甲基(-CH 2 SO 3 -)组并制备了带负电荷的配体bms 2-(2,2'-联吡啶-6,6'-二甲磺酸盐)和Ru-bms络合物[Ru(bms)(pic)2 ]。O–Ru–O咬合角从Ru-bda中的123°变为Ru-bms中的84° ,从而对催化行为产生了巨大影响。对反应中间体的系统分析表明,在Ce IV驱动的水氧化条件下,Ru-bms通过氧化分解一直转化为Ru-bda。
更新日期:2020-04-08
中文翻译:

Ru-bms配合物到Ru-bda的真实水氧化催化剂的结构演变:咬合角度很重要
钌基络合物在分子催化和机理研究方面都取得了进展。电子效应一直被认为是催化剂性能的重要因素,而对水氧化催化的咬合角效应的关注则较少。的Ru的BDA(的[Ru(BDA)(PIC)2 ]; BDA 2- = 2,2'-联吡啶-6,6'-二羧酸酯; PIC = 4-甲基吡啶)催化剂是最活跃的WOC之一,它具有严重变形的八面体结构,O–Ru–O咬合角为123°。在此,我们取代羧酸酯(-COO -)BDA的基团2-具有两个亚甲基(-CH 2 SO 3 -)组并制备了带负电荷的配体bms 2-(2,2'-联吡啶-6,6'-二甲磺酸盐)和Ru-bms络合物[Ru(bms)(pic)2 ]。O–Ru–O咬合角从Ru-bda中的123°变为Ru-bms中的84° ,从而对催化行为产生了巨大影响。对反应中间体的系统分析表明,在Ce IV驱动的水氧化条件下,Ru-bms通过氧化分解一直转化为Ru-bda。











































 京公网安备 11010802027423号
京公网安备 11010802027423号