当前位置:
X-MOL 学术
›
Cell Calcium
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
TRPM7 activation potentiates SOCE in enamel cells but requires ORAI.
Cell Calcium ( IF 4.3 ) Pub Date : 2020-02-28 , DOI: 10.1016/j.ceca.2020.102187 Guilherme H Souza Bomfim 1 , Veronica Costiniti 1 , Yi Li 1 , Youssef Idaghdour 2 , Rodrigo S Lacruz 1
Cell Calcium ( IF 4.3 ) Pub Date : 2020-02-28 , DOI: 10.1016/j.ceca.2020.102187 Guilherme H Souza Bomfim 1 , Veronica Costiniti 1 , Yi Li 1 , Youssef Idaghdour 2 , Rodrigo S Lacruz 1
Affiliation
|
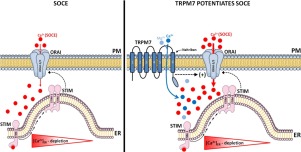
Calcium (Ca2+) release-activated Ca2+ (CRAC) channels mediated by STIM1/2 and ORAI (ORAI1-3) proteins form the dominant store-operated Ca2+ entry (SOCE) pathway in a wide variety of cells. Among these, the enamel-forming cells known as ameloblasts rely on CRAC channel function to enable Ca2+ influx, which is important for enamel mineralization. This key role of the CRAC channel is supported by human mutations and animal models lacking STIM1 and ORAI1, which results in enamel defects and hypomineralization. A number of recent reports have highlighted the role of the chanzyme TRPM7 (transient receptor potential melastanin 7), a transmembrane protein containing an ion channel permeable to divalent cations (Mg2+, Ca2+), as a modulator of SOCE. This raises the question as to whether TRPM7 should be considered an alternative route for Ca2+ influx, or if TRPM7 modifies CRAC channel activity in enamel cells. To address these questions, we monitored Ca2+ influx mediated by SOCE using the pharmacological TRPM7 activator naltriben and the inhibitor NS8593 in rat primary enamel cells and in the murine ameloblast cell line LS8 cells stimulated with thapsigargin. We also measured Ca2+ dynamics in ORAI1/2-deficient (shOrai1/2) LS8 cells and in cells with siRNA knock-down of Trpm7. We found that primary enamel cells stimulated with the TRPM7 activator potentiated Ca2+ influx via SOCE compared to control cells. However, blockade of TRPM7 with NS8593 did not decrease the SOCE peak. Furthermore, activation of TRPM7 in shOrai1/2 LS8 cells lacking SOCE failed to elicit Ca2+ influx, and Trpm7 knock-down had no effect on SOCE. Taken together, our data suggest that TRPM7 is a positive modulator of SOCE potentiating Ca2+ influx in enamel cells, but its function is fully dependent on the prior activation of the ORAI channels.
中文翻译:

TRPM7激活可增强搪瓷细胞中的SOCE,但需要ORAI。
由STIM1 / 2和ORAI(ORAI1-3)蛋白质介导的钙(Ca2 +)释放激活的Ca2 +(CRAC)通道在多种细胞中形成了主要的存储操纵性Ca2 +进入(SOCE)途径。其中,称为成釉细胞的搪瓷形成细胞依靠CRAC通道功能来使Ca2 +流入,这对于搪瓷矿化很重要。缺少STIM1和ORAI1的人类突变和动物模型支持了CRAC通道的这一关键作用,这会导致牙釉质缺陷和矿化不足。最近的许多报道都强调了杂合酶TRPM7(瞬态受体电位褪黑素7)的作用,该跨膜蛋白含有可渗透二价阳离子(Mg2 +,Ca2 +)的离子通道,作为SOCE的调节剂。这就提出了一个问题,即是否应将TRPM7视为Ca2 +流入的替代途径,或者TRPM7修改了釉质细胞中的CRAC通道活性。为了解决这些问题,我们使用药理学TRPM7活化剂纳特本和抑制剂NS8593监测了大鼠原发性釉质细胞和毒胡萝卜素刺激的鼠成釉细胞LS8细胞中由SOCE介导的Ca2 +内流。我们还测量了ORAI1 / 2缺乏(shOrai1 / 2)LS8细胞和siRNA敲低Trpm7的细胞中Ca2 +的动力学。我们发现,与对照细胞相比,用TRPM7活化剂刺激的原发性搪瓷细胞通过SOCE增强了Ca2 +的流入。但是,用NS8593阻断TRPM7不会降低SOCE峰。此外,缺少SOCE的shOrai1 / 2 LS8细胞中TRPM7的激活未能引起Ca2 +流入,而Trpm7的敲低对SOCE没有影响。在一起
更新日期:2020-02-28
中文翻译:

TRPM7激活可增强搪瓷细胞中的SOCE,但需要ORAI。
由STIM1 / 2和ORAI(ORAI1-3)蛋白质介导的钙(Ca2 +)释放激活的Ca2 +(CRAC)通道在多种细胞中形成了主要的存储操纵性Ca2 +进入(SOCE)途径。其中,称为成釉细胞的搪瓷形成细胞依靠CRAC通道功能来使Ca2 +流入,这对于搪瓷矿化很重要。缺少STIM1和ORAI1的人类突变和动物模型支持了CRAC通道的这一关键作用,这会导致牙釉质缺陷和矿化不足。最近的许多报道都强调了杂合酶TRPM7(瞬态受体电位褪黑素7)的作用,该跨膜蛋白含有可渗透二价阳离子(Mg2 +,Ca2 +)的离子通道,作为SOCE的调节剂。这就提出了一个问题,即是否应将TRPM7视为Ca2 +流入的替代途径,或者TRPM7修改了釉质细胞中的CRAC通道活性。为了解决这些问题,我们使用药理学TRPM7活化剂纳特本和抑制剂NS8593监测了大鼠原发性釉质细胞和毒胡萝卜素刺激的鼠成釉细胞LS8细胞中由SOCE介导的Ca2 +内流。我们还测量了ORAI1 / 2缺乏(shOrai1 / 2)LS8细胞和siRNA敲低Trpm7的细胞中Ca2 +的动力学。我们发现,与对照细胞相比,用TRPM7活化剂刺激的原发性搪瓷细胞通过SOCE增强了Ca2 +的流入。但是,用NS8593阻断TRPM7不会降低SOCE峰。此外,缺少SOCE的shOrai1 / 2 LS8细胞中TRPM7的激活未能引起Ca2 +流入,而Trpm7的敲低对SOCE没有影响。在一起











































 京公网安备 11010802027423号
京公网安备 11010802027423号