Journal of Controlled Release ( IF 10.5 ) Pub Date : 2020-02-28 , DOI: 10.1016/j.jconrel.2020.02.043 Rui Liu 1 , Yang An 2 , Wenfeng Jia 1 , Yushan Wang 1 , Yue Wu 3 , Yonghuan Zhen 4 , Jun Cao 5 , Huile Gao 1
|
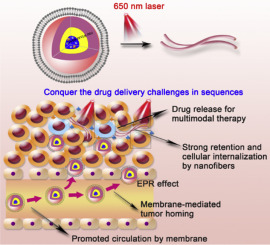
The current nanomedicines for cancer therapy based on the enhance permeability and retention (EPR) effect remain insufficient to satisfy the clinical need, and the challenges hindering nanomedicines delivery should be conquered for strong therapeutic efficacy. To address these problems, a membrane-coated laser-responsive shape changeable nanomedicine, I-P@NPs@M, is reported. The covering macrophage membrane promotes the circulation and tumor targeting of nanomedicines. Then the chlorin e6 (Ce6) in I-P@NPs@M can convert 650 nm laser into reactive oxygen species (ROS) to trigger the spherical micelles changing into nanofibers for strong retention in tumor region, consequently the linear nanofibers long locate and sustainably release drugs. On the other hand, the ROS not only directly kills tumor cells by photodynamic therapy but stimulates the dimeric paclitaxel (PTX) generating monomeric PTX. The combinational chemo- photodynamic therapy heavily suppresses tumor growth and inducing immunogenic cell death, which is synergistic with Indoximod (IND) inhibiting the IDO pathway to activate immune response for immunotherapy. By chemotherapy, photodynamic therapy and immunotherapy gathering, the treatment of I-P@NPs@M + laser shows the best antitumor effect, resulting in 85.27 ± 12.80% suppression of breast cancer in mice model, and also remarkably inhibits lung metastasis.
中文翻译:

巨噬细胞模拟形状可变的纳米药物保留在肿瘤中,用于乳腺癌的多峰治疗。
基于增强的渗透性和保留(EPR)效果的当前用于癌症治疗的纳米药物仍然不足以满足临床需求,并且应克服阻碍纳米药物递送的挑战以实现强大的治疗功效。为了解决这些问题,报道了一种膜涂覆的激光响应形状可变的纳米药物IP @ NPs @ M。覆盖的巨噬细胞膜促进纳米药物的循环和靶向肿瘤。然后IP @ NPs @ M中的二氢卟酚e6(Ce6)可以将650 nm激光转换为活性氧(ROS),触发球形胶束转变成纳米纤维,从而在肿瘤区域具有较强的滞留性,因此线性纳米纤维可以长期定位并可持续释放药物。另一方面,ROS不仅可以通过光动力疗法直接杀死肿瘤细胞,还可以刺激生成紫杉醇的二聚体单体PTX。化学光动力疗法的联合使用可极大地抑制肿瘤的生长并诱导免疫原性细胞死亡,这与吲哚昔莫德(IND)抑制IDO途径以激活免疫疗法的免疫反应具有协同作用。通过化学疗法,光动力疗法和免疫疗法的集会,IP @ NPs @ M +激光的治疗显示出最佳的抗肿瘤作用,从而在小鼠模型中抑制了85.27±12.80%的乳腺癌,并且还显着抑制了肺转移。与Indoximod(IND)协同抑制IDO途径激活免疫疗法的免疫治疗。通过化学疗法,光动力疗法和免疫疗法的集会,IP @ NPs @ M +激光的治疗显示出最佳的抗肿瘤作用,从而在小鼠模型中抑制了85.27±12.80%的乳腺癌,并且还显着抑制了肺转移。与Indoximod(IND)协同抑制IDO途径激活免疫疗法的免疫治疗。通过化学疗法,光动力疗法和免疫疗法的集会,IP @ NPs @ M +激光的治疗显示出最佳的抗肿瘤作用,从而在小鼠模型中抑制了85.27±12.80%的乳腺癌,并且还显着抑制了肺转移。











































 京公网安备 11010802027423号
京公网安备 11010802027423号