当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
α-Synuclein fibrillation products trigger the release of hexokinase I from mitochondria: Protection by curcumin, and possible role in pathogenesis of Parkinson's disease.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-02-27 , DOI: 10.1016/j.bbamem.2020.183251 Ziba Dehghani 1 , Ali Akbar Meratan 2 , Ali Akbar Saboury 1 , Mohsen Nemat-Gorgani 3
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-02-27 , DOI: 10.1016/j.bbamem.2020.183251 Ziba Dehghani 1 , Ali Akbar Meratan 2 , Ali Akbar Saboury 1 , Mohsen Nemat-Gorgani 3
Affiliation
|
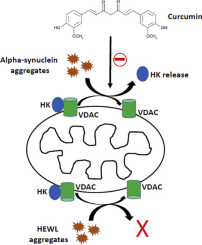
Extensive research has shown that assembling of α-synuclein amyloid aggregates on mitochondria is an important mechanistic feature of Parkinson's disease (PD) and other Lewy body disorders. However, the molecular mechanism(s) of its neuronal toxicity remain unclear. Type 1 Hexokinase (HKI), a key enzyme in the control of brain glucose metabolism, plays an important role in protecting against mitochondrially-regulated apoptosis through reducing generation of reactive oxygen species (ROS). The release of mitochondrially-bound HKI causes a significant decrease in enzyme activity and triggers oxidative stress. Here, we have investigated the potency of amyloid fibrillation products arising from α-synuclein and hen egg white lysozyme (HEWL) for the release of HKI and ROS content enhancement in mitochondria isolated from rat brain. Results clearly indicate the capacity of the fibrillation products of α-synuclein, and not HEWL, to trigger release of HKI from the Type A binding site of mitochondria for the enzyme and to induce mitochondrial ROS enhancement in a dose-dependent manner. Moreover, we found that curcumin was very effective in preventing mitochondrial HKI release and ROS enhancement induced by α-synuclein fibrillation products. The pathophysiological significance of mitochondrial HKI activity and localization in pathogenesis of neurodegenerative disorders including PD are discussed. Taken together, these results may offer insight into a possible mechanism by which disease-related peptides and proteins may exert their neuronal toxicity.
中文翻译:

α-突触核蛋白的纤颤产物触发线粒体中己糖激酶I的释放:姜黄素的保护作用,并可能在帕金森氏病的发病机理中发挥作用。
广泛的研究表明,在线粒体上组装α-突触核蛋白淀粉样蛋白聚集体是帕金森氏病(PD)和其他路易体病的重要机制。然而,其神经元毒性的分子机制仍不清楚。1型己糖激酶(HKI)是控制脑部葡萄糖代谢的关键酶,通过减少活性氧(ROS)的生成,在预防线粒体调节的细胞凋亡中起着重要作用。线粒体结合的HKI的释放导致酶活性显着降低并触发氧化应激。在这里,我们研究了由α-突触核蛋白和鸡蛋清溶菌酶(HEWL)产生的淀粉样蛋白原纤化产物对从大鼠脑中分离的线粒体中HKI释放和ROS含量增强的作用。结果清楚地表明,α-突触核蛋白而不是HEWL的原纤化产物能够触发线粒体A型结合位点的酶释放HKI,并以剂量依赖的方式诱导线粒体ROS增强。此外,我们发现姜黄素在预防由α-突触核蛋白原纤化产物诱导的线粒体HKI释放和ROS增强方面非常有效。讨论了线粒体HKI活性和局限性在包括PD在内的神经退行性疾病发病机理中的病理生理意义。综上所述,这些结果可以提供对疾病相关肽和蛋白质可能发挥其神经元毒性作用的可能机制的认识。触发HKI从线粒体的A型结合位点释放到该酶,并以剂量依赖的方式诱导线粒体ROS增强。此外,我们发现姜黄素在预防由α-突触核蛋白原纤化产物诱导的线粒体HKI释放和ROS增强方面非常有效。线粒体HKI活性和定位在包括PD的神经退行性疾病的发病机理中的病理生理意义进行了讨论。综上所述,这些结果可以提供对疾病相关肽和蛋白质可能发挥其神经元毒性作用的可能机制的见解。触发HKI从线粒体的A型结合位点释放到该酶,并以剂量依赖的方式诱导线粒体ROS增强。此外,我们发现姜黄素在预防由α-突触核蛋白原纤化产物诱导的线粒体HKI释放和ROS增强方面非常有效。讨论了线粒体HKI活性和局限性在包括PD在内的神经退行性疾病发病机理中的病理生理意义。综上所述,这些结果可以提供对疾病相关肽和蛋白质可能发挥其神经元毒性作用的可能机制的认识。我们发现姜黄素在预防由α-突触核蛋白原纤化产物诱导的线粒体HKI释放和ROS增强方面非常有效。讨论了线粒体HKI活性和局限性在包括PD在内的神经退行性疾病发病机理中的病理生理意义。综上所述,这些结果可以提供对疾病相关肽和蛋白质可能发挥其神经元毒性作用的可能机制的认识。我们发现姜黄素在预防由α-突触核蛋白原纤化产物诱导的线粒体HKI释放和ROS增强方面非常有效。讨论了线粒体HKI活性和局限性在包括PD在内的神经退行性疾病发病机理中的病理生理意义。综上所述,这些结果可以提供对疾病相关肽和蛋白质可能发挥其神经元毒性作用的可能机制的见解。
更新日期:2020-02-28
中文翻译:

α-突触核蛋白的纤颤产物触发线粒体中己糖激酶I的释放:姜黄素的保护作用,并可能在帕金森氏病的发病机理中发挥作用。
广泛的研究表明,在线粒体上组装α-突触核蛋白淀粉样蛋白聚集体是帕金森氏病(PD)和其他路易体病的重要机制。然而,其神经元毒性的分子机制仍不清楚。1型己糖激酶(HKI)是控制脑部葡萄糖代谢的关键酶,通过减少活性氧(ROS)的生成,在预防线粒体调节的细胞凋亡中起着重要作用。线粒体结合的HKI的释放导致酶活性显着降低并触发氧化应激。在这里,我们研究了由α-突触核蛋白和鸡蛋清溶菌酶(HEWL)产生的淀粉样蛋白原纤化产物对从大鼠脑中分离的线粒体中HKI释放和ROS含量增强的作用。结果清楚地表明,α-突触核蛋白而不是HEWL的原纤化产物能够触发线粒体A型结合位点的酶释放HKI,并以剂量依赖的方式诱导线粒体ROS增强。此外,我们发现姜黄素在预防由α-突触核蛋白原纤化产物诱导的线粒体HKI释放和ROS增强方面非常有效。讨论了线粒体HKI活性和局限性在包括PD在内的神经退行性疾病发病机理中的病理生理意义。综上所述,这些结果可以提供对疾病相关肽和蛋白质可能发挥其神经元毒性作用的可能机制的认识。触发HKI从线粒体的A型结合位点释放到该酶,并以剂量依赖的方式诱导线粒体ROS增强。此外,我们发现姜黄素在预防由α-突触核蛋白原纤化产物诱导的线粒体HKI释放和ROS增强方面非常有效。线粒体HKI活性和定位在包括PD的神经退行性疾病的发病机理中的病理生理意义进行了讨论。综上所述,这些结果可以提供对疾病相关肽和蛋白质可能发挥其神经元毒性作用的可能机制的见解。触发HKI从线粒体的A型结合位点释放到该酶,并以剂量依赖的方式诱导线粒体ROS增强。此外,我们发现姜黄素在预防由α-突触核蛋白原纤化产物诱导的线粒体HKI释放和ROS增强方面非常有效。讨论了线粒体HKI活性和局限性在包括PD在内的神经退行性疾病发病机理中的病理生理意义。综上所述,这些结果可以提供对疾病相关肽和蛋白质可能发挥其神经元毒性作用的可能机制的认识。我们发现姜黄素在预防由α-突触核蛋白原纤化产物诱导的线粒体HKI释放和ROS增强方面非常有效。讨论了线粒体HKI活性和局限性在包括PD在内的神经退行性疾病发病机理中的病理生理意义。综上所述,这些结果可以提供对疾病相关肽和蛋白质可能发挥其神经元毒性作用的可能机制的认识。我们发现姜黄素在预防由α-突触核蛋白原纤化产物诱导的线粒体HKI释放和ROS增强方面非常有效。讨论了线粒体HKI活性和局限性在包括PD在内的神经退行性疾病发病机理中的病理生理意义。综上所述,这些结果可以提供对疾病相关肽和蛋白质可能发挥其神经元毒性作用的可能机制的见解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号