Computational and Theoretical Chemistry ( IF 2.8 ) Pub Date : 2020-02-28 , DOI: 10.1016/j.comptc.2020.112766 Nasim Hassani
|
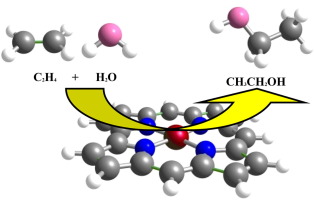
Since ethanol plays an important role in many fields of science, the adsorption and reaction mechanism of H2O and C2H4 to produce ethanol over three corrole complexes namely, CorM (M=B, Al, and Ga) have been theoretically studied employing density functional theory. In regard to the stable configuration of both H2O and C2H4 molecules on the CorM (M=B, Al, and Ga) surfaces, two routes for this reaction are considered, in which initially H2O or C2H4 adsorbed at the M/N4 active site of CorMs could participate in the catalytic hydration reaction of ethylene. Subsequently, both routes are studied via Eley–Rideal mechanism. It is predicted that the CorGa-based catalysts are a promising catalyst for hydration of ethylene, due to the lower energy barrier of the CorGa in comparison with the CorB/Al. This prediction suggests further experimental studies are required for catalytic hydration of ethylene over CorMs.
中文翻译:

乙烯在CorroleM(M = B,Al和Ga)配合物上的水合反应机理:一种理论方法
由于乙醇在许多科学领域中都起着重要作用,因此从理论上研究了H 2 O和C 2 H 4在三种Corro配合物(M = B,Al和Ga)上吸附和反应生成乙醇的机理。采用密度泛函理论 关于CorM(M = B,Al和Ga)表面上的H 2 O和C 2 H 4分子的稳定构型,考虑了该反应的两种途径,其中最初是H 2 O或C 2 H 4吸附在球茎的M / N4活性位点可能参与乙烯的催化水合反应。随后,研究了两条路线通过Eley-Rideal机制。据预测,由于CorGa基催化剂与CorB / Al相比具有较低的能垒,因此CorGa基催化剂是用于乙烯水合的有前途的催化剂。该预测表明乙烯在CorMs上催化水合还需要进一步的实验研究。



























 京公网安备 11010802027423号
京公网安备 11010802027423号