Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Mechanism for GSDMD Targeting by Autoprocessed Caspases in Pyroptosis.
Cell ( IF 45.5 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.cell.2020.02.002 Kun Wang 1 , Qi Sun 2 , Xiu Zhong 1 , Mengxue Zeng 3 , Huan Zeng 4 , Xuyan Shi 3 , Zilin Li 2 , Yupeng Wang 2 , Qiang Zhao 5 , Feng Shao 6 , Jingjin Ding 4
Cell ( IF 45.5 ) Pub Date : 2020-02-17 , DOI: 10.1016/j.cell.2020.02.002 Kun Wang 1 , Qi Sun 2 , Xiu Zhong 1 , Mengxue Zeng 3 , Huan Zeng 4 , Xuyan Shi 3 , Zilin Li 2 , Yupeng Wang 2 , Qiang Zhao 5 , Feng Shao 6 , Jingjin Ding 4
Affiliation
|
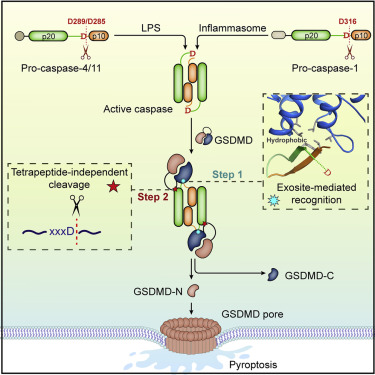
The pyroptosis execution protein GSDMD is cleaved by inflammasome-activated caspase-1 and LPS-activated caspase-11/4/5. The cleavage unmasks the pore-forming domain from GSDMD-C-terminal domain. How the caspases recognize GSDMD and its connection with caspase activation are unknown. Here, we show site-specific caspase-4/11 autoprocessing, generating a p10 product, is required and sufficient for cleaving GSDMD and inducing pyroptosis. The p10-form autoprocessed caspase-4/11 binds the GSDMD-C domain with a high affinity. Structural comparison of autoprocessed and unprocessed capase-11 identifies a β sheet induced by the autoprocessing. In caspase-4/11-GSDMD-C complex crystal structures, the β sheet organizes a hydrophobic GSDMD-binding interface that is only possible for p10-form caspase-4/11. The binding promotes dimerization-mediated caspase activation, rendering a cleavage independently of the cleavage-site tetrapeptide sequence. Crystal structure of caspase-1-GSDMD-C complex shows a similar GSDMD-recognition mode. Our study reveals an unprecedented substrate-targeting mechanism for caspases. The hydrophobic interface suggests an additional space for developing inhibitors specific for pyroptotic caspases.
中文翻译:

自动处理的胱天蛋白酶在焦磷酸中靶向GSDMD的结构机制。
炎性体激活的caspase-1和LPS激活的caspase-11 / 4/5裂解了热解执行蛋白GSDMD。切割从GSMDD-C-末端结构域解开孔形成结构域。caspase如何识别GSDMD及其与caspase激活的联系尚不清楚。在这里,我们显示了产生p10产物的特定于位点的caspase-4 / 11自动加工,对于裂解GSDMD和诱导发烧是必需的和足够的。p10形式的自动加工的caspase-4 / 11以高亲和力结合GSDMD-C结构域。自动处理和未处理的capase-11的结构比较可确定自动处理诱导的β折叠。在caspase-4 / 11-GSDMD-C复合晶体结构中,β片组织了疏水性GSDMD结合界面,只有p10形式的caspase-4 / 11才可能。结合促进二聚化介导的半胱天冬酶激活,使得切割独立于切割位点四肽序列。caspase-1-GSDMD-C复合物的晶体结构显示出相似的GSDMD识别模式。我们的研究揭示了针对胱天蛋白酶的前所未有的底物靶向机制。疏水性界面为开发特异于光解胱天蛋白酶的抑制剂提供了额外的空间。
更新日期:2020-02-28
中文翻译:

自动处理的胱天蛋白酶在焦磷酸中靶向GSDMD的结构机制。
炎性体激活的caspase-1和LPS激活的caspase-11 / 4/5裂解了热解执行蛋白GSDMD。切割从GSMDD-C-末端结构域解开孔形成结构域。caspase如何识别GSDMD及其与caspase激活的联系尚不清楚。在这里,我们显示了产生p10产物的特定于位点的caspase-4 / 11自动加工,对于裂解GSDMD和诱导发烧是必需的和足够的。p10形式的自动加工的caspase-4 / 11以高亲和力结合GSDMD-C结构域。自动处理和未处理的capase-11的结构比较可确定自动处理诱导的β折叠。在caspase-4 / 11-GSDMD-C复合晶体结构中,β片组织了疏水性GSDMD结合界面,只有p10形式的caspase-4 / 11才可能。结合促进二聚化介导的半胱天冬酶激活,使得切割独立于切割位点四肽序列。caspase-1-GSDMD-C复合物的晶体结构显示出相似的GSDMD识别模式。我们的研究揭示了针对胱天蛋白酶的前所未有的底物靶向机制。疏水性界面为开发特异于光解胱天蛋白酶的抑制剂提供了额外的空间。











































 京公网安备 11010802027423号
京公网安备 11010802027423号