Electrochemistry Communications ( IF 4.7 ) Pub Date : 2020-02-27 , DOI: 10.1016/j.elecom.2020.106703 Peter Ó. Conghaile , Rakesh Kumar , Maria Luisa Ferrer , Dónal Leech
|
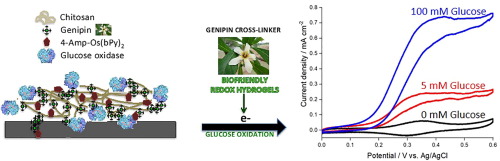
Genipin is used to simultaneously crosslink chitosan, amine-containing osmium redox complex and glucose oxidase on graphite disks to produce enzyme-based electrodes for glucose oxidation. The enzyme electrodes produce glucose oxidation current densities of 730 µAcm−2 in 50 mM phosphate buffered saline (150 mM NaCl, pH 7.4, 37°C) containing 100 mM glucose at an applied potential of 0.45 V (vs. Ag/AgCl), higher than the 440 µAcm−2 for conventional poly(ethylene glycol) diglycidyl ether (PEGDGE) epoxy ring-opening cross-linked films. Addition of multiwalled carbon nanotubes results in current response as high as 4.9 ± 0.3 mAcm−2 in 100 mM glucose. The genipin cross-linked hydrogels deliver a 3 fold increase in stability for continuous amperometric current production over a 20 hour period when compared to PEGDGE cross-linked hydrogels. Genipin provides an effective route for simultaneous crosslinking glucose oxidase, chitosan and the redox complex while further optimisation of the crosslinking process holds promise for application of the enzyme electrodes as fuel cell and sensor devices.
中文翻译:

通过酶电极进行的葡萄糖氧化,使用Genipin交联壳聚糖,葡萄糖氧化酶和含胺的氧化还原络合物
Genipin用于同时交联壳聚糖,含胺的氧化还原络合物和葡萄糖盘上的葡萄糖氧化酶,以产生用于葡萄糖氧化的基于酶的电极。酶电极在含有100 mM葡萄糖的50 mM磷酸盐缓冲盐水(150 mM NaCl,pH 7.4,37 °C)中产生的葡萄糖氧化电流密度为730 µAcm -2,施加的电势为0.45 V(vs。Ag / AgCl),高于常规聚乙二醇二缩水甘油醚(PEGDGE)环氧开环交联膜的440 µAcm -2。添加多壁碳纳米管会导致电流响应高达4.9±0.3 mAcm -2在100 mM葡萄糖中。与PEGDGE交联水凝胶相比,京尼平交联水凝胶的稳定性提高了3倍,可在20小时内连续产生安培电流。Genipin提供了同时交联葡萄糖氧化酶,壳聚糖和氧化还原复合物的有效途径,而交联过程的进一步优化则有望将酶电极用作燃料电池和传感器设备。











































 京公网安备 11010802027423号
京公网安备 11010802027423号