Tetrahedron ( IF 2.1 ) Pub Date : 2020-02-27 , DOI: 10.1016/j.tet.2020.131087 Masaya Mori , Yuuta Fujikawa , Hideshi Inoue
|
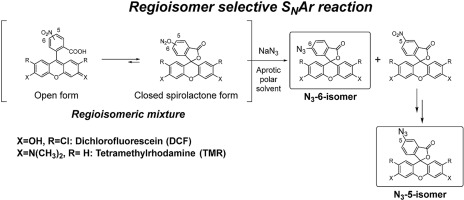
In this study, we present an efficient and general strategy for the individual preparation of both isomers of 5(6)-functionalized xanthene-based fluorophores. Spectroscopic analysis of the acid-base equilibrium of 5(6)-nitro xanthene dyes has shown that they exist predominantly in the colorless spirolactone form in certain aprotic dipolar solvents. In such solvents, regioisomerically selective ipso-substitution of the nitro group by sodium azide occurs at the 6- position but not at the 5- position due to the electron-withdrawing spirolactone moiety at the para-position, relative to the nitro group. This reaction allows the separation of the isomer with the 6-azide group and the intact 5-nitro isomer. The 5-nitro group of the latter was then reduced to an amino group and subsequently converted to an azide group. This strategy enables the preparation of both the 5- and 6-functionalized isomers individually from a mixture of precursors, which is otherwise unachievable. The azide isomers were then compared in reactivity by strain-promoted azide-alkyne cycloaddition (SPAAC) with bicycle[6.1.0]non-4-yene (BCN).
中文翻译:

通过S N Ar反应方便地合成区域异构纯的5和6-官能化的x吨染料,并比较它们对点击反应的反应性
在这项研究中,我们为5(6)-功能化的x吨基荧光团的两种异构体的单独制备提供了一种有效的通用策略。5(6)-硝基x吨染料的酸碱平衡的光谱分析表明,它们在某些质子惰性偶极溶剂中主要以无色螺内酯形式存在。在这样的溶剂中,结构异构体选择性的本位由叠氮化钠的硝基3'-取代发生在6-位,但不是在5-位由于在吸电子基团螺内酯对-相对于硝基的位置。该反应允许分离具有6-叠氮基的异构体和完整的5-硝基异构体。然后将后者的5-硝基还原为氨基,然后转化为叠氮基。该策略使得能够从前体的混合物中分别制备5-和6-官能化的异构体,否则这是无法实现的。然后通过应变促进的叠氮化物-炔烃环加成反应(SPAAC)与bicycle [6.1.0] non-4-yene(BCN)比较叠氮化物异构体的反应性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号