当前位置:
X-MOL 学术
›
Pharmacol. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein Engineering in the Ubiquitin System: Tools for Discovery and Beyond.
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2020-04-01 , DOI: 10.1124/pr.118.015651 Bo Zhao 1 , Yien Che Tsai 2 , Bo Jin 2 , Bufan Wang 2 , Yiyang Wang 2 , Han Zhou 2 , Tomaya Carpenter 2 , Allan M Weissman 1 , Jun Yin 1
Pharmacological Reviews ( IF 19.3 ) Pub Date : 2020-04-01 , DOI: 10.1124/pr.118.015651 Bo Zhao 1 , Yien Che Tsai 2 , Bo Jin 2 , Bufan Wang 2 , Yiyang Wang 2 , Han Zhou 2 , Tomaya Carpenter 2 , Allan M Weissman 1 , Jun Yin 1
Affiliation
|
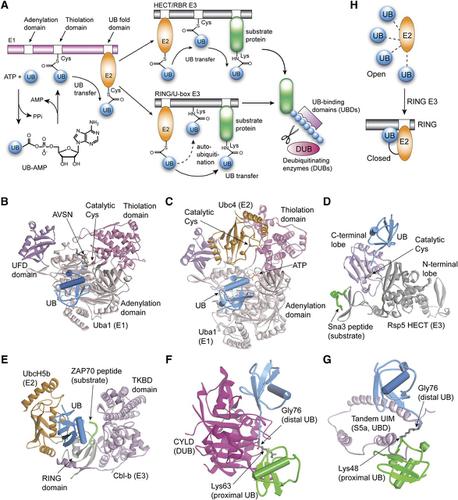
Ubiquitin (UB) transfer cascades consisting of E1, E2, and E3 enzymes constitute a complex network that regulates a myriad of biologic processes by modifying protein substrates. Deubiquitinating enzymes (DUBs) reverse UB modifications or trim UB chains of diverse linkages. Additionally, many cellular proteins carry UB-binding domains (UBDs) that translate the signals encoded in UB chains to target proteins for degradation by proteasomes or in autophagosomes, as well as affect nonproteolytic outcomes such as kinase activation, DNA repair, and transcriptional regulation. Dysregulation of the UB transfer pathways and malfunctions of DUBs and UBDs play causative roles in the development of many diseases. A greater understanding of the mechanism of UB chain assembly and the signals encoded in UB chains should aid in our understanding of disease pathogenesis and guide the development of novel therapeutics. The recent flourish of protein-engineering approaches such as unnatural amino acid incorporation, protein semisynthesis by expressed protein ligation, and high throughput selection by phage and yeast cell surface display has generated designer proteins as powerful tools to interrogate cell signaling mediated by protein ubiquitination. In this study, we highlight recent achievements of protein engineering on mapping, probing, and manipulating UB transfer in the cell. SIGNIFICANCE STATEMENT: The post-translational modification of proteins with ubiquitin alters the fate and function of proteins in diverse ways. Protein engineering is fundamentally transforming research in this area, providing new mechanistic insights and allowing for the exploration of concepts that can potentially be applied to therapeutic intervention.
中文翻译:

泛素系统中的蛋白质工程:发现及其他工具。
泛素 (UB) 转移级联由 E1、E2 和 E3 酶组成,构成一个复杂的网络,通过修饰蛋白质底物来调节无数的生物过程。去泛素化酶 (DUB) 可逆转 UB 修饰或修剪不同连接的 UB 链。此外,许多细胞蛋白携带 UB 结合域 (UBD),将 UB 链中编码的信号翻译为目标蛋白,以便被蛋白酶体或自噬体降解,并影响非蛋白水解结果,例如激酶激活、DNA 修复和转录调节。 UB 转移途径的失调以及 DUB 和 UBD 的功能障碍在许多疾病的发展中发挥着致病作用。更好地了解 UB 链组装机制和 UB 链编码的信号应有助于我们了解疾病发病机制并指导新疗法的开发。最近蛋白质工程方法的蓬勃发展,例如非天然氨基酸掺入、通过表达蛋白质连接进行的蛋白质半合成以及通过噬菌体和酵母细胞表面展示的高通量选择,已经产生了设计蛋白质作为询问蛋白质泛素化介导的细胞信号传导的强大工具。在这项研究中,我们重点介绍了蛋白质工程在细胞内定位、探测和操纵 UB 转移方面的最新成就。意义陈述:泛素对蛋白质的翻译后修饰以多种方式改变蛋白质的命运和功能。蛋白质工程正在从根本上改变这一领域的研究,提供新的机制见解,并允许探索可能应用于治疗干预的概念。
更新日期:2020-04-01
中文翻译:

泛素系统中的蛋白质工程:发现及其他工具。
泛素 (UB) 转移级联由 E1、E2 和 E3 酶组成,构成一个复杂的网络,通过修饰蛋白质底物来调节无数的生物过程。去泛素化酶 (DUB) 可逆转 UB 修饰或修剪不同连接的 UB 链。此外,许多细胞蛋白携带 UB 结合域 (UBD),将 UB 链中编码的信号翻译为目标蛋白,以便被蛋白酶体或自噬体降解,并影响非蛋白水解结果,例如激酶激活、DNA 修复和转录调节。 UB 转移途径的失调以及 DUB 和 UBD 的功能障碍在许多疾病的发展中发挥着致病作用。更好地了解 UB 链组装机制和 UB 链编码的信号应有助于我们了解疾病发病机制并指导新疗法的开发。最近蛋白质工程方法的蓬勃发展,例如非天然氨基酸掺入、通过表达蛋白质连接进行的蛋白质半合成以及通过噬菌体和酵母细胞表面展示的高通量选择,已经产生了设计蛋白质作为询问蛋白质泛素化介导的细胞信号传导的强大工具。在这项研究中,我们重点介绍了蛋白质工程在细胞内定位、探测和操纵 UB 转移方面的最新成就。意义陈述:泛素对蛋白质的翻译后修饰以多种方式改变蛋白质的命运和功能。蛋白质工程正在从根本上改变这一领域的研究,提供新的机制见解,并允许探索可能应用于治疗干预的概念。











































 京公网安备 11010802027423号
京公网安备 11010802027423号