当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Assessment of Biological Role and Insight into Druggability of the Plasmodium falciparum Protease Plasmepsin V.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-02-27 , DOI: 10.1021/acsinfecdis.9b00460 Alexander J Polino 1 , Armiyaw S Nasamu 1 , Jacquin C Niles 2 , Daniel E Goldberg 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-02-27 , DOI: 10.1021/acsinfecdis.9b00460 Alexander J Polino 1 , Armiyaw S Nasamu 1 , Jacquin C Niles 2 , Daniel E Goldberg 1
Affiliation
|
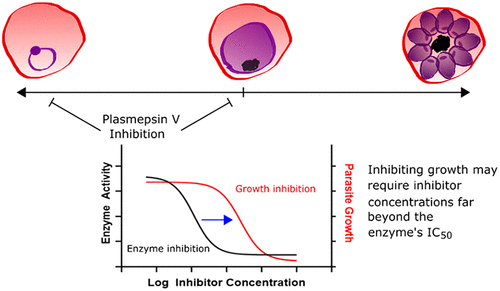
Upon infecting a red blood cell (RBC), the malaria parasite Plasmodium falciparum drastically remodels its host by exporting hundreds of proteins into the RBC cytosol. This protein export program is essential for parasite survival. Hence export-related proteins could be potential drug targets. One essential enzyme in this pathway is plasmepsin V (PMV), an aspartic protease that processes export-destined proteins in the parasite endoplasmic reticulum (ER) at the Plasmodium export element (PEXEL) motif. Despite long-standing interest in this enzyme, functional studies have been hindered by the inability of previous technologies to produce a regulatable lethal depletion of PMV. To overcome this technical barrier, we designed a system for stringent post-transcriptional regulation allowing a tightly controlled, tunable knockdown of PMV. Using this system, we found that PMV must be dramatically depleted to affect parasite growth, suggesting the parasite maintains this enzyme in substantial excess. Surprisingly, depletion of PMV arrested parasite growth immediately after RBC invasion, significantly before the death from exported protein deficit that has previously been described. The data suggest that PMV inhibitors can halt parasite growth at two distinct points in the parasite life cycle. However, overcoming the functional excess of PMV in the parasite may require inhibitor concentrations far beyond the enzyme's IC50.
中文翻译:

恶性疟原虫蛋白酶 Plasmepsin V 的生物学作用评估和成药性洞察。
感染红细胞 (RBC) 后,疟原虫恶性疟原虫通过将数百种蛋白质输出到红细胞胞质中,彻底改造其宿主。这种蛋白质输出程序对于寄生虫的生存至关重要。因此,与出口相关的蛋白质可能是潜在的药物靶点。该途径中的一种重要酶是疟原虫蛋白酶 V (PMV),它是一种天冬氨酸蛋白酶,可处理寄生虫内质网 (ER) 中疟原虫输出元件 (PEXEL) 基序处的输出目的地蛋白质。尽管长期以来人们对这种酶很感兴趣,但由于以前的技术无法产生可调节的 PMV 致死消耗,功能研究受到了阻碍。为了克服这一技术障碍,我们设计了一种严格的转录后调控系统,可以严格控制、可调节的 PMV 敲低。使用该系统,我们发现 PMV 必须急剧消耗才能影响寄生虫的生长,这表明寄生虫使这种酶保持在大量过量状态。令人惊讶的是,PMV 的消耗在红细胞入侵后立即阻止了寄生虫的生长,明显早于先前描述的因输出蛋白质缺陷而死亡。数据表明,PMV 抑制剂可以在寄生虫生命周期的两个不同点阻止寄生虫生长。然而,克服寄生虫中 PMV 的功能过剩可能需要远远超过该酶 IC50 的抑制剂浓度。
更新日期:2020-02-18
中文翻译:

恶性疟原虫蛋白酶 Plasmepsin V 的生物学作用评估和成药性洞察。
感染红细胞 (RBC) 后,疟原虫恶性疟原虫通过将数百种蛋白质输出到红细胞胞质中,彻底改造其宿主。这种蛋白质输出程序对于寄生虫的生存至关重要。因此,与出口相关的蛋白质可能是潜在的药物靶点。该途径中的一种重要酶是疟原虫蛋白酶 V (PMV),它是一种天冬氨酸蛋白酶,可处理寄生虫内质网 (ER) 中疟原虫输出元件 (PEXEL) 基序处的输出目的地蛋白质。尽管长期以来人们对这种酶很感兴趣,但由于以前的技术无法产生可调节的 PMV 致死消耗,功能研究受到了阻碍。为了克服这一技术障碍,我们设计了一种严格的转录后调控系统,可以严格控制、可调节的 PMV 敲低。使用该系统,我们发现 PMV 必须急剧消耗才能影响寄生虫的生长,这表明寄生虫使这种酶保持在大量过量状态。令人惊讶的是,PMV 的消耗在红细胞入侵后立即阻止了寄生虫的生长,明显早于先前描述的因输出蛋白质缺陷而死亡。数据表明,PMV 抑制剂可以在寄生虫生命周期的两个不同点阻止寄生虫生长。然而,克服寄生虫中 PMV 的功能过剩可能需要远远超过该酶 IC50 的抑制剂浓度。











































 京公网安备 11010802027423号
京公网安备 11010802027423号