当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
P-Stereogenic Amino-Phosphines as Chiral Ligands: From Privileged Intermediates to Asymmetric Catalysis.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-02-27 , DOI: 10.1021/acs.accounts.9b00633 Albert Cabré 1, 2 , Antoni Riera 1, 2 , Xavier Verdaguer 1, 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-02-27 , DOI: 10.1021/acs.accounts.9b00633 Albert Cabré 1, 2 , Antoni Riera 1, 2 , Xavier Verdaguer 1, 2
Affiliation
|
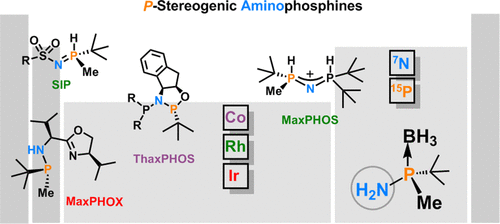
Among chiral phosphines, P-stereogenic phosphines provide unparalleled activity and selectivity and have thus emerged as "state-of-the-art" ligands for asymmetric hydrogenation and other industrially relevant processes. However, the synthesis of this type of ligand implies lengthy multistep sequences, which are a hurdle for many laboratories. There is a lack of methods for the rapid construction of P-stereogenic phosphine ligands. In this respect, P-stereogenic synthons that can be rapidly incorporated into a given ligand scaffold are highly desirable. Over the last 10 years, our group has unveiled that P-stereogenic aminophosphines can be rapidly assembled in a convenient fashion from the corresponding primary aminophosphine and/or the corresponding phosphinous acid.Using cis-1-amino-2-indanol as chiral auxiliary, we devised a multigram synthesis of tert-butylmethylaminophosphine borane and tert-butylmethylphosphinous acid borane, which are key intermediate synthons. Primary aminophosphine works as nucleophilic intermediates at nitrogen. From this synthon, aminodiphosphine (MaxPHOS) and secondary imino phosphoranes (SIP) ligands were synthesized. These ligands exhibit a tautomeric equilibrium between the PH and NH forms, and because of that, they do not undergo oxidation in air. NH/PH tautomerism does not jeopardize their configurational stability, and most importantly, in the presence of a metal source, the equilibrium is shifted toward the NH form, thus allowing coordination through phosphorus. Rh-MaxPHOS and Rh-SIP complexes have been used in asymmetric hydrogenation and [2 + 2 + 2] cycloaddition reactions with outstanding results. On the other hand, P-stereogenic phosphinous acid, upon activation, serves as an electrophilic reagent with amine nucleophiles, allowing SN2 reactions at phosphorus with complete inversion of configuration. This coupling technology exhibits a great potential because it allows the incorporation of the P*-phosphine fragment in numerous ligand structures, provided there is an amino group with which to react. In a mild and efficient process, phosphinous acid has been coupled to hydrazine to yield C2 diphosphines and to chiral benzoimidazole-amines to yield P-stereogenic benzoimidazole-phosphine ligands. The most powerful ligand system, however, arises from the condensation of three independent fragments: our phosphinous acid borane, an amino acid, and an amino alcohol, which yields a library of phosphino-oxazoline ligands named MaxPHOX. The corresponding Ir-MaxPHOX catalyst library was applied with excellent results in the asymmetric hydrogenation of α,β-unsaturated esters, 2-aryl allyl phthalimides, unfunctionalized tetrasubstituted alkenes, cyclic enamides, and N-aryl and N-methyl imines. It also has found application in asymmetric isomerization of alkenes.Overall, we developed key P-stereogenic building blocks that can be incorporated stereospecifically to ligand scaffolds and demonstrated that integration of the P*-aminophosphine fragment in a given catalytic system provides structural diversity that can be a critical contribution to obtaining optimal results and selectivity.
中文翻译:

P-Stereogenic氨基膦作为手性配体:从特权中间体到不对称催化。
在手性膦中,P-立体异构膦提供无与伦比的活性和选择性,因此已成为不对称氢化和其他工业相关工艺的“最先进”配体。然而,这种类型的配体的合成意味着冗长的多步序列,这是许多实验室的障碍。缺乏快速构建P-立体异构膦配体的方法。在这方面,非常需要能够快速掺入给定配体支架中的P-立体异构合成子。在过去的10年中,我们的研究小组发现,可以方便地从相应的伯氨基膦和/或相应的次膦酸中快速组装P-stereogenic氨基膦。使用顺式1-氨基-2-茚满醇作为手性助剂,我们设计了叔丁基甲基氨基膦硼烷和叔丁基甲基次膦酸硼烷的多谱合成法,它们是关键的中间合成子。伯氨基膦在氮上作为亲核中间体。由该合成子合成氨基二膦(MaxPHOS)和仲亚氨基膦(SIP)配体。这些配体在PH和NH形式之间表现出互变异构平衡,因此,它们在空气中不会发生氧化。NH / PH互变异构不会损害其构型稳定性,最重要的是,在存在金属源的情况下,平衡会朝NH形式转移,从而允许通过磷进行配位。Rh-MaxPHOS和Rh-SIP配合物已用于不对称氢化和[2 + 2 + 2]环加成反应中,具有出色的结果。另一方面,活化后,对位立体亚膦酸与胺亲核试剂一起用作亲电子试剂,使磷中的SN2反应完全颠倒构型。该偶联技术显示出巨大的潜力,因为它允许P *-膦片段掺入许多配体结构中,前提是存在一个可与之反应的氨基。在温和而有效的过程中,亚膦酸已与肼偶合,生成C2二膦,并与手性苯并咪唑-胺偶合,生成P-立体异构苯并咪唑-膦配体。然而,最强大的配体系统来自三个独立片段的缩合:我们的次膦酸硼烷,一种氨基酸和一种氨基醇,这产生了一个名为MaxPHOX的膦基-恶唑啉配体库。相应的Ir-MaxPHOX催化剂库在α,β-不饱和酯,2-芳基烯丙基邻苯二甲酰亚胺,未官能化的四取代烯烃,环状酰胺和N-芳基和N-甲基亚胺的不对称氢化中获得了优异的结果。总体而言,我们开发了关键的P-stereogenic构建基,可以将其立体定向地掺入配体支架中,并证明了在给定的催化体系中P *-氨基膦片段的整合提供了结构多样性,可以对获得最佳结果和选择性至关重要。和N-芳基和N-甲基亚胺。总体而言,我们开发了关键的P-stereogenic构建基,可以将其立体定向地掺入配体支架中,并证明了在给定的催化体系中P *-氨基膦片段的整合提供了结构多样性,可以对获得最佳结果和选择性至关重要。和N-芳基和N-甲基亚胺。总体而言,我们开发了关键的P-stereogenic构建基,可以将其立体定向地掺入配体支架中,并证明了在给定的催化体系中P *-氨基膦片段的整合提供了结构多样性,可以对获得最佳结果和选择性至关重要。
更新日期:2020-02-28
中文翻译:

P-Stereogenic氨基膦作为手性配体:从特权中间体到不对称催化。
在手性膦中,P-立体异构膦提供无与伦比的活性和选择性,因此已成为不对称氢化和其他工业相关工艺的“最先进”配体。然而,这种类型的配体的合成意味着冗长的多步序列,这是许多实验室的障碍。缺乏快速构建P-立体异构膦配体的方法。在这方面,非常需要能够快速掺入给定配体支架中的P-立体异构合成子。在过去的10年中,我们的研究小组发现,可以方便地从相应的伯氨基膦和/或相应的次膦酸中快速组装P-stereogenic氨基膦。使用顺式1-氨基-2-茚满醇作为手性助剂,我们设计了叔丁基甲基氨基膦硼烷和叔丁基甲基次膦酸硼烷的多谱合成法,它们是关键的中间合成子。伯氨基膦在氮上作为亲核中间体。由该合成子合成氨基二膦(MaxPHOS)和仲亚氨基膦(SIP)配体。这些配体在PH和NH形式之间表现出互变异构平衡,因此,它们在空气中不会发生氧化。NH / PH互变异构不会损害其构型稳定性,最重要的是,在存在金属源的情况下,平衡会朝NH形式转移,从而允许通过磷进行配位。Rh-MaxPHOS和Rh-SIP配合物已用于不对称氢化和[2 + 2 + 2]环加成反应中,具有出色的结果。另一方面,活化后,对位立体亚膦酸与胺亲核试剂一起用作亲电子试剂,使磷中的SN2反应完全颠倒构型。该偶联技术显示出巨大的潜力,因为它允许P *-膦片段掺入许多配体结构中,前提是存在一个可与之反应的氨基。在温和而有效的过程中,亚膦酸已与肼偶合,生成C2二膦,并与手性苯并咪唑-胺偶合,生成P-立体异构苯并咪唑-膦配体。然而,最强大的配体系统来自三个独立片段的缩合:我们的次膦酸硼烷,一种氨基酸和一种氨基醇,这产生了一个名为MaxPHOX的膦基-恶唑啉配体库。相应的Ir-MaxPHOX催化剂库在α,β-不饱和酯,2-芳基烯丙基邻苯二甲酰亚胺,未官能化的四取代烯烃,环状酰胺和N-芳基和N-甲基亚胺的不对称氢化中获得了优异的结果。总体而言,我们开发了关键的P-stereogenic构建基,可以将其立体定向地掺入配体支架中,并证明了在给定的催化体系中P *-氨基膦片段的整合提供了结构多样性,可以对获得最佳结果和选择性至关重要。和N-芳基和N-甲基亚胺。总体而言,我们开发了关键的P-stereogenic构建基,可以将其立体定向地掺入配体支架中,并证明了在给定的催化体系中P *-氨基膦片段的整合提供了结构多样性,可以对获得最佳结果和选择性至关重要。和N-芳基和N-甲基亚胺。总体而言,我们开发了关键的P-stereogenic构建基,可以将其立体定向地掺入配体支架中,并证明了在给定的催化体系中P *-氨基膦片段的整合提供了结构多样性,可以对获得最佳结果和选择性至关重要。









































 京公网安备 11010802027423号
京公网安备 11010802027423号