当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the emulsion copolymerization kinetics of vinyl acetate and vinyl silanes
Polymer Chemistry ( IF 4.1 ) Pub Date : 2020/02/27 , DOI: 10.1039/d0py00126k Aitor Barquero 1, 2, 3, 4, 5 , Fernando Ruipérez 1, 2, 3, 4, 5 , María Jesús Barandiaran 1, 2, 3, 4, 5 , Jose Ramon Leiza 1, 2, 3, 4, 5
Polymer Chemistry ( IF 4.1 ) Pub Date : 2020/02/27 , DOI: 10.1039/d0py00126k Aitor Barquero 1, 2, 3, 4, 5 , Fernando Ruipérez 1, 2, 3, 4, 5 , María Jesús Barandiaran 1, 2, 3, 4, 5 , Jose Ramon Leiza 1, 2, 3, 4, 5
Affiliation
|
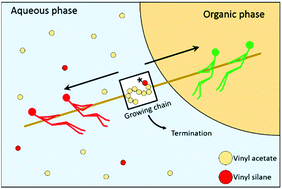
The emulsion copolymerization between vinyl acetate (VAc) and vinyl alkoxysilanes is an interesting yet not well-understood process. In the present work, the copolymerization between VAc and vinyl trimethoxysilane (VTMS) is taken as a model system and studied using different polymerization techniques in order to understand the underlying mechanism that governs the emulsion polymerization kinetics. The solution and bulk copolymerization kinetics can be explained by the reactivity ratios of the monomers determined in this work (rVTMS = 0, rVAc = 0.211). However, the emulsion copolymerization kinetics presents features that cannot be explained by the reactivity ratios, as VTMS concentrations above 5 wt% stopped the emulsion polymerization when initiated with hydrophilic initiators (such as persulfates). This was a consequence of the slow propagating radicals terminating in the aqueous phase, decreasing the concentration of surface-active radicals and thus radical entry. When more hydrophobic vinyl alkoxysilanes such as vinyl triethoxysilane are used, the inhibiting effect was less prominent, because the silane concentration in the aqueous phase was smaller. Using Density Functional Theory calculations it was found that the hydrolyzed version of the alkoxysilane monomers had a different reactivity ratio.
中文翻译:

了解乙酸乙烯酯和乙烯基硅烷的乳液共聚动力学
乙酸乙烯酯(VAc)与乙烯基烷氧基硅烷之间的乳液共聚是一个有趣的但尚未得到很好理解的方法。在目前的工作中,VAc和乙烯基三甲氧基硅烷(VTMS)之间的共聚被作为模型系统,并使用不同的聚合技术进行研究,以便了解控制乳液聚合动力学的基本机理。溶液和本体共聚动力学可以通过这项工作中确定的单体的反应率来解释(r VTMS = 0,r VAc= 0.211)。然而,乳液共聚动力学具有不能用反应性比率解释的特征,因为当由亲水性引发剂(例如过硫酸盐)引发时,高于5重量%的VTMS浓度停止乳液聚合。这是由于缓慢增长的自由基在水相中终止,降低了表面活性自由基的浓度,从而降低了自由基的进入的结果。当使用更多的疏水性乙烯基烷氧基硅烷如乙烯基三乙氧基硅烷时,由于水相中的硅烷浓度较小,因此抑制效果不太明显。使用密度泛函理论计算发现,烷氧基硅烷单体的水解形式具有不同的反应率。
更新日期:2020-03-31
中文翻译:

了解乙酸乙烯酯和乙烯基硅烷的乳液共聚动力学
乙酸乙烯酯(VAc)与乙烯基烷氧基硅烷之间的乳液共聚是一个有趣的但尚未得到很好理解的方法。在目前的工作中,VAc和乙烯基三甲氧基硅烷(VTMS)之间的共聚被作为模型系统,并使用不同的聚合技术进行研究,以便了解控制乳液聚合动力学的基本机理。溶液和本体共聚动力学可以通过这项工作中确定的单体的反应率来解释(r VTMS = 0,r VAc= 0.211)。然而,乳液共聚动力学具有不能用反应性比率解释的特征,因为当由亲水性引发剂(例如过硫酸盐)引发时,高于5重量%的VTMS浓度停止乳液聚合。这是由于缓慢增长的自由基在水相中终止,降低了表面活性自由基的浓度,从而降低了自由基的进入的结果。当使用更多的疏水性乙烯基烷氧基硅烷如乙烯基三乙氧基硅烷时,由于水相中的硅烷浓度较小,因此抑制效果不太明显。使用密度泛函理论计算发现,烷氧基硅烷单体的水解形式具有不同的反应率。











































 京公网安备 11010802027423号
京公网安备 11010802027423号